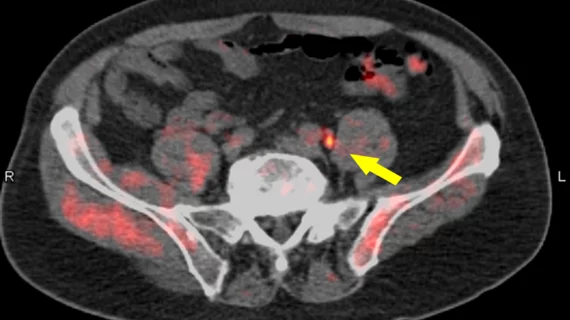
Fluciclovine PET/CT detects previously unseen lesions in prostate cancer patients
18F-Fluciclovine PET/CT can help detect previously undetected lesions in patients with biochemical recurrence of prostate cancer, according to a report presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2018 Annual Meeting.
“Recurrent prostate cancer poses an important medical challenge,” Austin R. Pantel, MD, of the University of Pennsylvania in Philadelphia, said in a prepared statement. “Currently approved anatomical imaging procedures have limitations in identifying the sites of recurrence of prostate cancer after definitive treatment. This can make decision-making difficult when assessing these patients.”
Pantel added that up to 30 percent of prostate cancer patients “will develop local or distant recurrence within 10 years of radical prostatectomy or radiation therapy.”
For the clinical trial, researchers evaluated 213 male patients with recurrent prostate cancer with 18F-fluciclovine PET/CT after “conventional imaging” resulted in negative or indefinite results. Fifty-nine percent of those patients had their clinical management changed as a direct result of findings from the 18F-Fluciclovine PET/CT imaging. Seventy-eight percent of those changes were considered “major.”
“While investigation of the long-term clinical outcomes of these changes in management is warranted, these results indicate, for the first time in a prospective U.S. study, that decisions based on 18F-fluciclovine PET/CT imaging may facilitate more appropriate management in men with biochemical recurrence of their prostate cancer,” Pantel said in the statement. “The study also demonstrates the broad potential applicability of 18F-fluciclovine PET/CT imaging across a range of clinical settings.”