Gadolinium Risk Management: 3 Pillars for a Sound Strategy
As debates continue over the nature and causal link between the administration of gadolinium and a host of symptoms and signs termed gadolinium deposition disease (GDD), radiologists and their partner institutions would be unwise to ignore the very real risk of litigation that the concerns are driving. After all, more than a few malpractice suits have been advanced on easily understood emotion over hard-to-parse scientific evidence. And it’s better to be cautious now than to face a preventable lawsuit later.
How to prepare? Before we get to that, let’s quickly review what brought us to where we are now with gadolinium.
GDD has been described based on a reported correlation between a nonspecific constellation of symptoms and exposure to gadolinium-based contrast agents (GBCAs) for MR imaging. Deposition of gadolinium in tissues including the brain, skin, bone and kidneys after GBCA exposure has been proposed as the cause of GDD. Diagnostic criteria include a series of symptoms, mostly subjective, that may or may not be temporally related to GBCA administration.
Unlike nephrogenic systemic fibrosis (NSF), which is now a well described entity—it’s primarily associated with the use of gadolinium in patients with renal impairment—GDD is primarily described in patients without currently recognized risk factors.
In late 2017, the FDA issued a warning for all nine approved GBCAs to make physicians aware that gadolinium can remain in the body for months to years after an administration. The following spring, the agency mandated that its Medication Guide on gadolinium be provided to all patients receiving a GBCA.
Meanwhile European regulators have taken a stronger stance, suspending the use of all Class I GBCAs, known as linear agents, which are associated with increased gadolinium retention. European regulators also recommended that gadolinium be used only when necessary and in the lowest doses possible. (For more on this, see “Gadolinium: Actual Offender or Unwitting Pretender?”)
Fortunately, based on current information, the risk of harm from GDD seems extremely low. The severity of the morbidity is not catastrophic and, to date, no one has invoked death as a sequela of GDD.
Still, while causality and a definite relationship between GDD and GBCAs has not been conclusively proven, the American College of Radiology and other groups have called for ongoing gadolinium research.
3 Pillars, 1 Game Plan
As a risk manager, I remain quite concerned about GDD. Regardless of the subjective nature of the symptomatology and relatively loose relationship to the time of GBCA administration, the actions of European regulators and the FDA raise legitimate liability concerns.
Based on the current knowledge of GDD and the medical malpractice atmosphere around it, imaging centers and health systems would be prudent to immediately implement a risk mitigation strategy. This should be built on three fundamental pillars:
1. Appropriate use. The use of GBCAs for a large array of MRI indications is clearly within the standard of care. Nonetheless, given the present uncertainty regarding the risks associated with gadolinium retention, GBCA usage should be reserved for when it is clinically necessary.
Stated another way, GBCA use should be reserved for cases in which the risk of missing disease by not performing the MRI with contrast is significant. This may warrant switching to a non-contrast technique or even another imaging modality when the pre-test probability for disease is low.
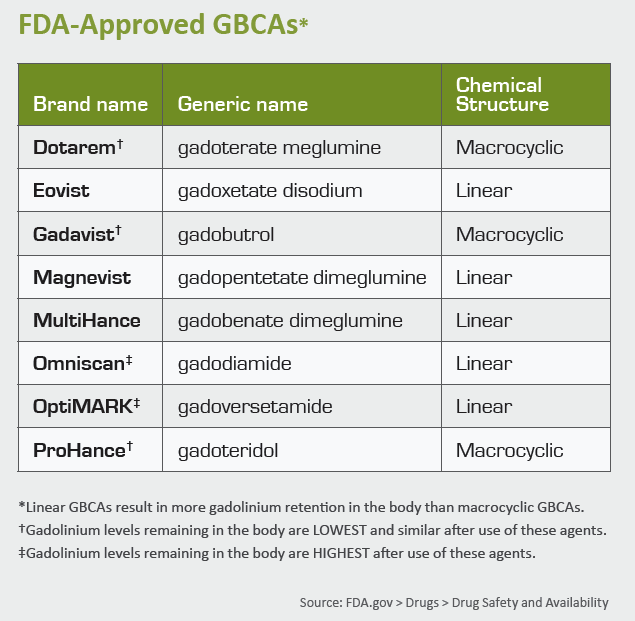
2. Optimal GBCA selection. Although a causal link between a particular type or class of GBCA and GDD has not been conclusively established, health systems should nonetheless remain cautious. Following the lead of European regulators, many U.S. providers have favored use of class II GBCAs (macrocyclic agents) over class I GBCAs (linear agents). But to achieve true risk mitigation, providers must go one step further by only administering the class II GBCA associated with the least gadolinium retention.
Based on the available data, gadoteridol (tradename: Prohance) is associated with the least gadolinium retention, particularly in the weeks following GBCA administration, depositing less than half the gadolinium in the brain compared to the other class II GBCAs.
3. Patient education. If we are to maximize patient autonomy and participation in decision-making, we must ensure that every patient receiving a GBCA is informed and educated about issues surrounding gadolinium retention. The FDA has taken an important first step by mandating that patients be provided a printed guide prior to receiving a GBCA.
Since much remains unknown about GDD, prudent risk management also should include written patient consent preceded by a full explanation of risks, benefits and alternatives. Both shared decision-making and patient engagement have been shown to enhance patient satisfaction and will likely minimize possible litigation.
In conclusion, recently publicized safety concerns related to retained gadolinium from GBCAs remain a subject of continuing research. Nonetheless, the potential for lawsuits aimed at radiologists or health systems related to GDD remains significant, particularly given the large patient populations exposed to GBCAs and the subjectivity of the disease symptomology.
During this time of uncertainty, the best risk management approach is to:
- Use GBCAs only when clinically indicated and at optimized doses;
- Preferentially use gadoteridol (Prohance) unless there are clear reasons to choose an alternative GBCA with higher gadolinium retention; and
- Ensure that adequate patient education is in place, including considering the use of informed consent or other shared decision-making strategies.
Dr. Argy is a radiologist and attorney who is a recognized expert in radiology risk management and patient safety. He’s online at NicolasArgy.com.
Related MRI Contrast Agent Safety Content:
A deep dive into gadolinium-based adverse reactions
Allergic reactions to iodinated CT contrast increase likelihood of sensitivity to GBCAs
Researchers detail data on gadolinium-related adverse reactions
Radiologists must take a data-driven approach to discuss gadolinium, mitigate liability risk
Radiologists see potential to reduce GBCA administration with new synthetic MRI technique
Gadolinium-based contrast agents are safe, even at higher doses, new research suggests
Gadolinium debate rages on, with radiologist questioning recent GBCA liability guidance
ACR committee proposes new term for symptoms associated with gadolinium exposure
Closing the knowledge gap on gadolinium retention risks
Radiologists find direct evidence linking gadolinium-based contrast agent to higher retention rates
AI software that eliminates need for gadolinium contrast during imaging exams wins patent
Research may offer new method to detect GBCA on MRI
Radiology, other multispecialty groups urge caution with GBCAs during interventional pain procedures
Cardiac MRI contrast agents are low-risk and safe for ‘overwhelming’ majority of patients
Health orgs publish special report about gadolinium retention, GBCA use in imaging
Rodent brains retain gadolinium after repeated administration of GBCA a year after injection
Advanced MRI mapping spots traces of gadolinium in the brain invisible during conventional scanning
Radiologists should keep patients’ best interests in mind to mitigate gadolinium liability risk
