The FDA has approved a CT guidance system for interventional radiologists performing percutaneous procedures with robotics. And the European Union has greenlit a family of mobile CT scanners.
In the U.S., Xact Robotics says FDA cleared its Ace Xtend remote control unit. This lets interventionalists avoid CT radiation while using the imaging to precisely steer instruments from the control room during such procedures as ablations, drainages and biopsies.
“Interventional radiology is among the medical specialties that are facing a physician shortage in the coming years, and technologies that can improve their efficiencies are crucial,” says Jeffrey Solomon, MD, vice president of Medical Affairs at Xact. “By equipping different users with tools that can standardize the procedure and potentially shorten procedure times, we can maximize the number of patients they see and help to mitigate the gap between the patient population and the physician population.”
Xact’s announcement is here.
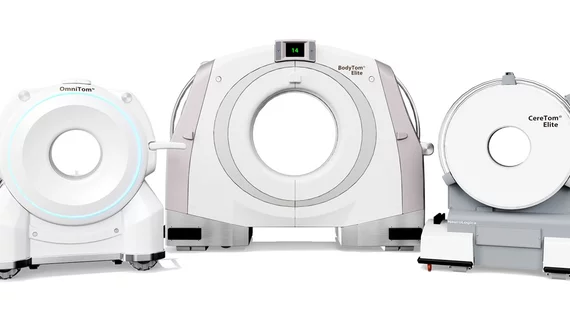
In Europe, Samsung subsidiary NeuroLogica says the three scanners in its Elite Mobile CT line have earned CE marking for complying with current EU medical device regulations. The scanners are named OmniTom Elite, BodyTom Elite and CereTom Elite.
NeuroLogica points out that the EU’s newish Medical Device Regulation (MDR), which replaces the former Medical Device Directive (MDD), places a strong emphasis on technical documentation, clinical data and postmarket surveillance.
NeuroLogica already complies with the U.S. FDA quality system regulations and is certified to ISO 13485 standard, according to Ninad Gujar, PhD, MBA, the company’s VP of regulatory affairs.
“Compliance with EU MDR is an important regulatory milestone that exhibits NeuroLogica’s efforts towards demonstrating conformance to EU regulatory requirements, making mobile CT systems available in the European Economic Area and remaining committed to product quality and safety,” Gujar says. “Our products and processes comply with global regulatory requirements allowing design, manufacturing, installation, service and engineering of imaging systems for medical applications.”
The NeuroLogica announcement is here.