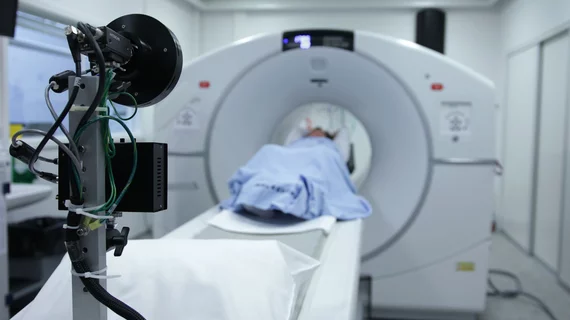
FDA greenlights expansion of CT-guided, robotically supported IR system
Xact Robotics has been cleared to add robotically supported ablation to the interventional radiology procedures for which its branded Ace system previously received FDA’s OK.
Xact says Ace has been used clinically in more than 200 other kinds of CT-guided percutaneous procedures.
The company says the approval for ablations will allow interventional radiologists to perform these operations with nonlinear steering that facilitates high accuracy and average skin-to-target times under nine minutes.
“Ablation procedures are challenging to many users in terms of the accuracy and time required to place the ablation probes,” says Harel Gadot, Xact’s founder and executive chairman, in a May 18 announcement. “The proven ability of our system to reach relatively small targets, regardless of target movement or obstacles, with unparalleled accuracy and one insertion to target will provide better patient outcomes with greater efficiencies.”
Privately held Xact Robotics has offices in Hingham, Mass., and Caesarea, Israel.