A molecular contrast agent previously cleared for use during surgery for ovarian cancer may now be used in similar fashion during lung operations.
Pafolacianine, marketed as Cytalux by On Target Laboratories of West Lafayette, Ind., received the ovarian cancer go-ahead in November 2021 and approval for the expanded indication this month.
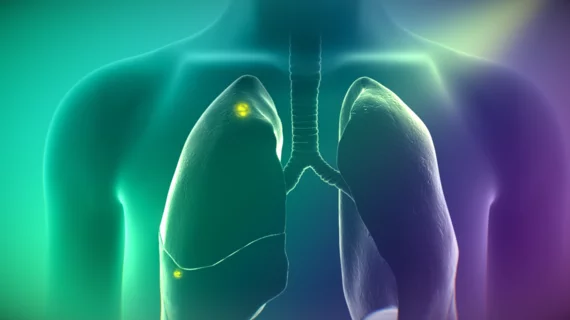
Following injection by standard IV prior to surgery, the drug works with a near-infrared fluorescence imaging system to show surgeons suspicious lesions that may have been missed in preoperative imaging.
In its announcement of the new FDA clearance, On Target quotes Linda Martin, MD, thoracic surgery chief at the University of Virginia School of Medicine.
Martin says Cytalux demonstrated sufficient safety and efficacy in a clinical trial that it “has potential to become standard of care in thoracic surgery.”
On Target says Cytalux works by binding to folate receptors, which are often overexpressed in certain cancers. Under fluorescent light, the drug gleams to highlight such bindings.
“To date, there have been limited ways for surgeons to confidently assess the location and full extent of cancerous tissue while operating,” the company says. “Intraoperative molecular imaging (IMI) is an emerging category of technology for surgical oncology in which targeted imaging agents are injected into patients to highlight cancer cells making them visible during surgery.”
Full announcement with multimedia content here.