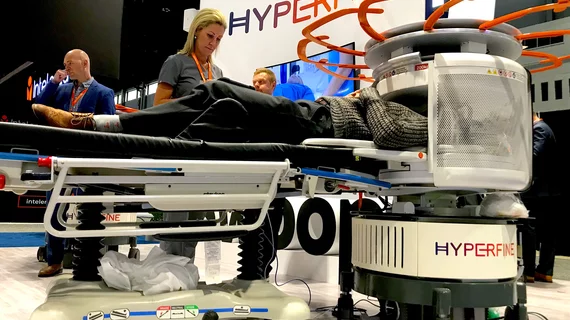
FDA clears new artificial intelligence capabilities for portable MRI scanner
The U.S. Food and Drug Administration has granted 510(k) clearance for new artificial intelligence capabilities utilized by Hyperfine’s portable MRI scanner, the company announced Monday.
Following the update, the Swoop system now offers expanded AI denoising capabilities, incorporating advanced post-processing into the imaging sequence. This will allow for crisper images and facilitate more accurate diagnoses, the Guilford, Connecticut-based manufacturer said Oct. 9.
“Our eighth FDA software clearance in three years for the Swoop system underscores our relentless drive for innovation and continuous improvement,” Hyperfine President and CEO Maria Sainz said in an announcement. “Our focus remains on providing quality brain imaging to more providers and patients in more sites of care.”
Sainz and colleagues said Hyperfine plans to roll out the updated system software “in the coming months.” The FDA first cleared the Swoop system in 2020 as a portable MR brain imaging device for producing scans that display the internal structure of the head (where a full diagnostic exam is not clinically practical).