FDA clears augmented reality platform that creates 3D models via CT scans
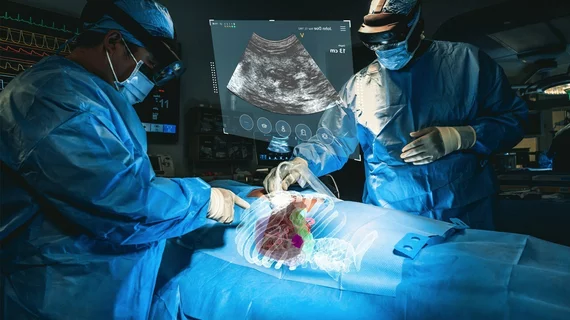
The U.S. Food and Drug Administration has cleared a new augmented reality platform that utilizes CT scans to project 3D images on patients during minimally invasive procedures.
Cleveland-based MedView XR Inc. announced the news on Thursday, noting the “long-standing limitations” of current 2D medical imaging. Its XR90 device overcomes these limits by creating models of the patient’s anatomy combined with live ultrasound to help physicians perform biopsies or tumor ablations.
The company also recently scored $15 million in funding from organizations including the Mayo and Cleveland clinics and GE Healthcare.
“This is not only the first 510(k) clearance for MediView, but it is the first 510(k) clearance for an augmented reality device utilizing live imaging combined with 3D XR visualization for pre- and intra-operative indications…,” Adam Cargill, director of quality, regulatory and clinical affairs, said in a July 20 announcement. This, he added, “sets the stage for further advancements in augmented reality in the healthcare space.”
MediView said its technology also allows clinicians in remote locations to collaborate in real time via shared visualization and communication. Such features provide increased support for understaffed facilities and those located in remote areas, the company noted.