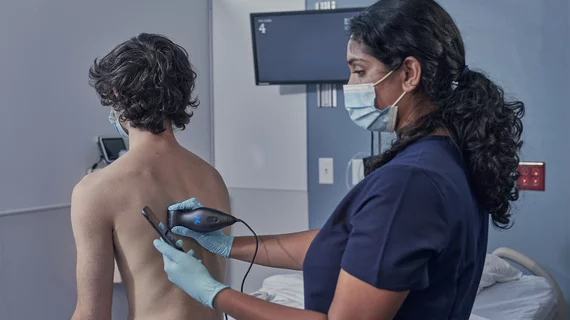
FDA clears new point-of-care ultrasound system from Butterfly Network
The U.S. Food and Drug Administration has cleared the newest point-of-care ultrasound system from Butterfly Network, the Burlington, Massachusetts, company announced Monday.
Butterfly iQ3 is the third generation of its semiconductor-based, single-probe, whole-body scanner. Features will include a new ergonomic design, faster data-processing speeds and optimized image resolution.
Leaders plan to reveal further details about the system on Thursday at the 42nd Annual J.P. Morgan Healthcare Conference, taking place in San Francisco.
Shares of the publicly traded company—founded in 2011 by scientist and entrepreneur Jonathan Rothberg, PhD—were up 22% Monday on news of the FDA clearance.