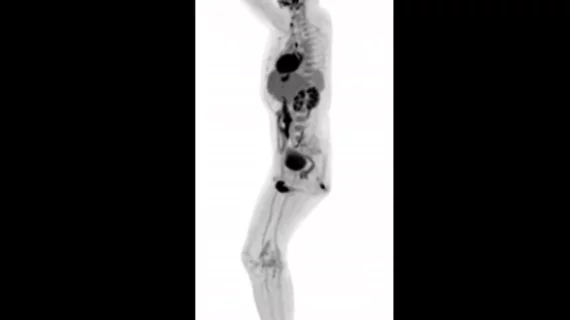
Shanghai, China-based United Imaging Healthcare announced Tuesday, Jan. 22, that its uEXPLORER total-body scanner has received FDA clearance.
uEXPLORER is the first imaging solution that captures a 3D picture of the patient’s entire body from a single position. Initial images from the PET/CT scanner were unveiled at RSNA 2018 in Chicago.
“This is truly a game-changing, revolutionary day for the advancement of medical technology,” Jeffrey M. Bundy, PhD, CEO of United Imaging Healthcare Solutions, said in a prepared statement. “The human images the uEXPLORER produces have been nothing short of astonishing, especially when one considers the very short acquisition times. This device will bring us into completely new areas of discussion with our customers, as we jointly re-think the role of PET in the North American healthcare system. We are thrilled to be able to start sharing it widely in the U.S.”
“As we had originally envisioned the uEXPLORER will have a profound impact on clinical research and patient care,” Simon Cherry, PhD, a professor at the University of California, Davis, and co-developer of uEXPLORER, said in the same statement.