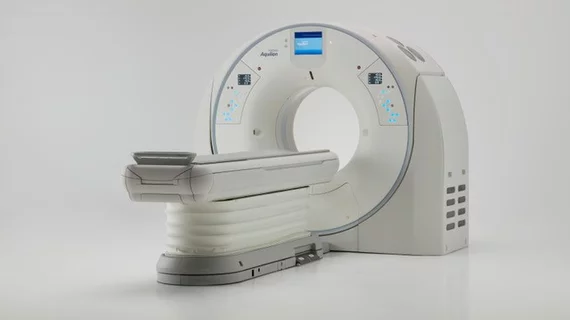
FDA clears Canon’s ‘ultra-high resolution’ CT system
Tustin, California-based Canon Medical Systems USA announced April 9 that it’s new Aquilion Precision ultra-high-resolution CT system has been cleared by the FDA. According to the company release, the system can improve resolution of traditional CT exams, being able to resolve anatomy as small as 150 microns.
“The increased amount of information delivered by the Aquilion Precision opens new doors for healthcare providers,” said Dominic Smith, senior director of CT, PET/CT and MR Business Units for Canon Medical Systems USA. “The system delivers higher resolution images than other systems on the market, enabling customers to deliver better patient care.”
The system includes dose efficiency with detector channels that are 0.25 mm. It also includes improved scintillator quantum efficiency and detector circuitry.