Can cold-cathode X-ray combined with teleradiology and AI eliminate health disparities?
The Israeli medical imaging vendor Nanox says it has a vision for the future of healthcare to address health disparities and lack of access to care. It envisions a new business model and plans to leverage a package of new technologies, including cold-cathode X-ray technology to help reduce costs, coupled with a new and inexpensive imaging system that combines teleradiology with artificial intelligence (AI). The business model is to enable any clinic or hospital in the developing world or rural areas to access its technology and no upfront costs using a pay-per-exam fee. The exams will be read by remote teleradiologists, including subspecialists, and AI will help augment clinical staff and radiologists to offer additional health screenings for all patients scanned.
After a few years of talk, the vendor now appears on the edge of making this a reality. But the tipping point will be regulatory approval in Europe and the U.S. for its innovative new imaging technology.
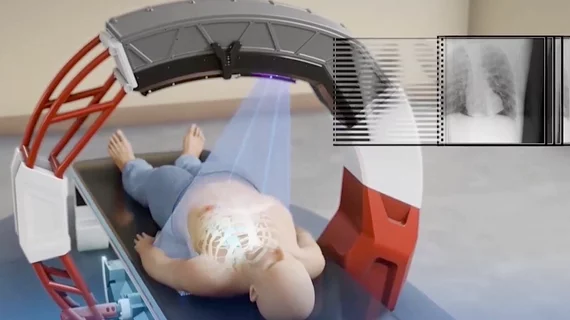
The company's plan is to offer its Nanox ARC multi-source 3D tomosynthesis X-ray system to centers in developing countries in Africa, Asia and South America using the pay-per-scan model, rather than having clinics and hospitals buy the systems as a capital investment. The exam datasets would be transferred via secure web connections to teleradiology radiologists located elsewhere in the world for reads. The system also will have multiple artificial intelligence (AI) algorithms running in the background to help with a more comprehensive health screening that can be added automatically to the radiology reports.
Nanox said the AI and the use of teleradiology are being offered as a solution to the large shortage of radiologists in the developing world. The low-cost machines also would enable wider access to medical imaging. Two-thirds of the world's population in developing countries do not have meaningful access to medical imaging, and Nanox says existing medical imaging systems are too expensive and complex for mass deployment. The company's business plan is to deploy 15,000 ARC units by the end of 2024, provided the company gains regulatory approval. Nanox says it hopes to become tone of the largest medical imaging networks in the world over the next few years, starting with deployment in areas that have the greatest need for medical imaging and better access to care.
In November 2021, Nanox closed more than $200 million in acquisitions, purchasing artificial intelligence company Zebra Medical Vision and teleradiology company USARad, which employs more than 300 radiologists. Nanox said these companies helped build out its capability to develop AI applications and to create a global teleradiology program. The vendor already has two FDA clearances for its AI applications, and a third will be submitted for review by mid-2023. They just need regulatory approval off their imaging system to put the plan in motion.
The Nanox ARC may offer more advanced imaging than standard X-ray
The company says the key is its Nanox ARC multi-source 3D tomosynthesis X-ray system, currently pending U.S. Food and Drug Administration (FDA) 510(k) review. This system uses cold-cathode X-ray technology, which by itself would make this system revolutionary. But the system also will offer multiple X-ray sources to create a series of images in an arc around the patient that can be reconstructed into cone-beam computed tomography (CT) images, or 3D tomosynthesis imaging. This technology is best known for its growing use with 3D mammography systems to enable looking at slices, or layers, of the anatomy similar to a CT dataset. This makes diagnosis more precise because a radiologist can scroll though the slices of anatomy rather than looking at all the anatomy in one 2D slice with lots of overlapping structures.
The ARC uses very lightweight construction of a patient bed and a small circular frame around the table and patient bed. This holds the X-ray tubes and digital detectors. Because of it uses cold-cathode tube technology, most of the usual weight on X-ray systems in the form of cooling systems and the structural support metal to stabilize these systems and the imaging platform has been eliminated. This should greatly reduce production costs, weight and savings on shipping and installation.
Advantages of a tomosynthesis system is that more advanced, CT-like exams can be performed compared to basic 2D X-rays, offering more detailed clinical information. Examples of clinical imaging from the ARC were shown during press event Nov. 16, including had and foot images where they showed how scrolling through the slices in the too image can help verify if there is a small fracture or if it is just the edge of an overlapping bone.
The Nanox cold-cathode X-ray tube technology
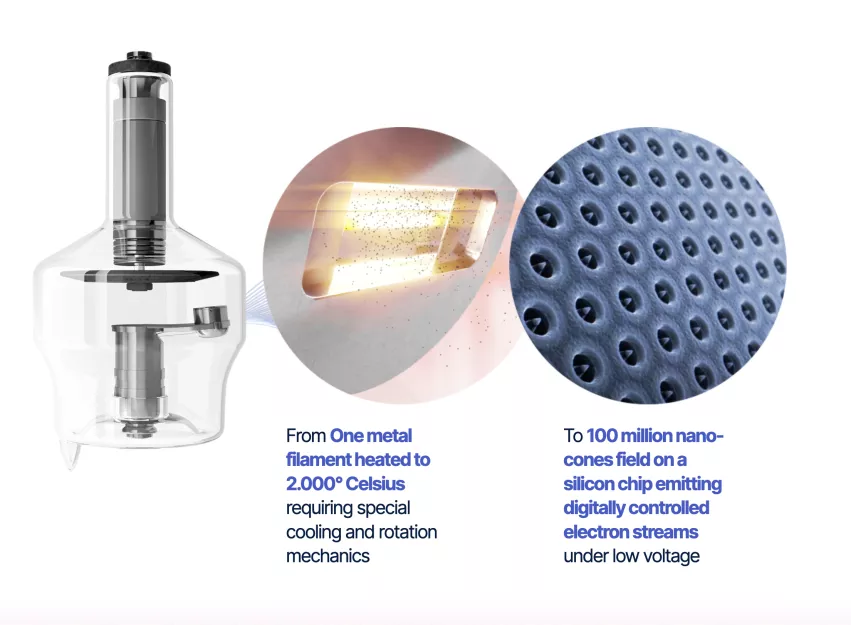
The Nanox Source X-ray tube uses a semiconductor chip that has 100 million nano-cones embedded on the silicon chip, rather that using a metal target anode. This allows emitting digitally controlled electron streams under low voltage. Nanox opened a semiconductor chip fabrication plant in South Korea in April 2022 to produce the cold-cathode tube components.
This new type of X-ray tube also maintains a low temperature because there is no heat associated with electrons exiting the chip, as compared to traditional X-ray tubes. X-ray tube technology that has been used for the past century uses an electron beam hitting a metal target to generate a small percentage of X-ray energy and 90% or more heat energy when the tube is on. To handle this excessive heat, passive and/or active cooling systems are required all X-ray systems (digital X-ray, CT, angiography). This requires extra structural metal and increased weight on X-ray systems, which can be eliminated using cold-cathode technology.
Nanox said its X-ray tube’s expected operating range is between 20 and 120 kV. The company also hopes the low cost of the tubes, about $100, will help proliferate the use of and adoption of the inexpensive Nanox X-ray systems.
The cold-cathode technology was discussed and demonstrated amid great fan fare at the 2020 virtual RSNA meeting. However, there have been hiccups along the way since 2020.
While cold-cathode technology has existed for several years in research centers, even on the first commercialized cold-cathode DRX Revolution Nano mobile X-ray system made by Carestream, the microscopic cone structures used in the tubes are known to be brittle and start to breakdown from use over a relatively short amount of time, as compared to conventional tubes. This causes degradation on the imaging and requires tube replacement on a more regular basis. Durability has been the key problem with cold-cathode technology, which has prevented it from replacing traditional work-horse X-ray anode tube technology.
Nanox ignored repeated media requests for interviews, updates and additional information on its cold-cathode technology following RSNA 2020 and in 2021. Carestream also did not want to discuss its cold-cathode technology when contacted for an interview. The Nano disappeared from Carestream's front of RSNA booth display after a couple years. The Nano has been upgraded in the past couple years and is still offered for sale for specialty application in emergency rooms, use where size, weight and mobility are important.
If Nanox can produce its cold-cathode tubes for around $100 each and its technology still requires more frequent tube replacements, it may not matter because of the low tube cost.
Legal questions raised on misrepresentation of the Nanox technology
In 2021, the U.S. Securities and Exchange Commission investigated Nanox business practices amid allegations from investors that they were duped. Investors accused the company of misrepresentations of its technology and for not having an operational ARC prototype system in use anywhere. During a company earnings call in November 2021, Nanox admitted it spent about $600,000 on legal fees related to the SEC inquiry and proposed class-action lawsuit brought by investors.
Nanox did received the FDA clearance for its single-source Nanox Cart X-ray system in April 2021. It submitted its multi-source Nanox ARC 3D tomosynthesis X-ray system for FDA review in 2021, but the FDA found deficiencies in the application.
In a Nanox investor's meeting call Nov. 10, the company announced it made a new submission to the FDA in late September 2022 for its multi-source Nanox ARC system. The company said it is also continuing to pursue European CE Mark approval of the system
First clinical Nanox ARC units ready to deploy soon after regulatory approval
Nanox CEO Erez Meltzer said it remains the goal of the company to put its first ARC system demonstration unit into clinical service in Nigeria by the end of 2022, pending regulatory clearances. Once approvals are received, he said the first systems will be shipped and it begin begin training users.
"Globally, we have a total of 6,850 preordered units. We believe that once we begin to deploy the multisource Nanox ARC in Nigeria and in other early adopting countries, health system hospitals and distributors will see the service that the Nanox.ARC provides," Meltzer said on the call.
AI to augment radiologists and aid in preventive care
Nanox gained an artificial intelligence division when it purchased Zebra Medical Imaging in 2021. Zebra already had made inroads in the AI market prior to the acquisition.
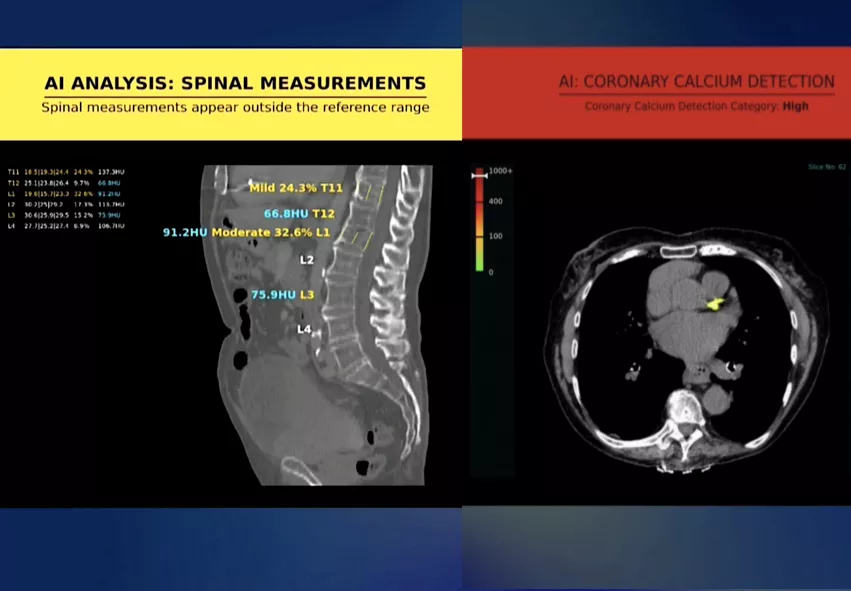
The company already has two FDA-cleared algorithms with CPT codes to detect coronary calcium an a bone solution to detect vertebral compression fractures and low bone mineral density. Both of these are designed to to operate in the background for every chest and abdominal scan as a virtual physical exam. A third AI algorithm for fatty liver assessment is undergoing testing and the company hopes to submit for FDA in mid-2023.
Research on these algorithms was conducted by Perry Pickhardt, MD, professor of radiology, chief, gastrointestinal imaging, director of cancer imaging, University of Wisconsin School of Medicine and Public Health, who spoke at the Nov. 16 press conference. He said this type of AI technology has the promise to greatly enhance personalized medicine by giving risk assessments for several diseases at once for a specific patient as incidental findings that are not always noted and rarely follow up on, even if they are included in a radiology report.
"This superpowers the CT incidental CT image data in all scans we perform, which have largely been ignored, now with AI algorithms we can harness this valuable information, which we have basically disregarded up until now. This is sometimes referred to as opportunistic CT scanning, which leverages CT data unrelated to reason for the current exam," Pickhardt explained.
He said the AI can automatically look at all the imaging data unrelated to the reason for a scan, such as virtual CT colonoscopy, or scans to determine if a patient has a kidney stone. Pickhardt said he sees this as a future direction for AI to help move medicine more toward preventive care, rather than reacting after a disease has progressed and is symptomatic and there is less that can be done to roll back the clock in a disease.
He showed examples of patient colonoscopy exams where the bone density AI showed the patient was at high risk for a bone fracture. He showed serial CT scans of the same patient over several years where the bone AI algorithm was applied and it clearly showed the bone density dropping with each scan. He said if this technology existed in the past, interventions could have ben done years before this patient ended up in the hospital with a catastrophic hip fracture.
Nanox working with U.S. IDNs on research
It is not just developing countries Nanox is working with. In the U.S., Nanox is working with a few IDNs on research projects using its technology, including Northwell and Spectrum Health.
"Northwell, I would say, is more than just an IDN. They are kind of strategic IDN that we work with, that we jointly develop some applications, are developing some business models and intended use of the AI applications. They are planning to adopt and install all the applications that we have," Meltzer explained.
Nanox is not exhibiting at RSNA 2022.