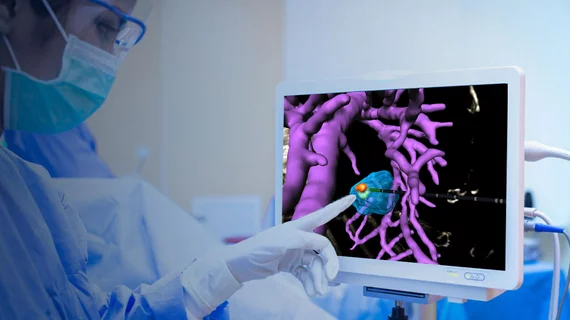
FDA clears AI software for aiding interventional radiologists during image-guided procedures
The U.S. Food and Drug Administration has granted 510(k) clearance for new artificial intelligence-powered software to aid interventional radiologists in planning liver ablation procedures.
Israel-based developer Techsomed announced the news Monday, noting that its VisAble.IO product can increase treatment precision by helping physicians confirm ablation zones. The product is part of its BioTrace solution, which leverages ultrasound, CT or MRI to provide real-time visualization during procedures.
“We are excited to receive 510(k) clearance for our VisAble.IO solution and appreciate the relentless efforts of our entire team in achieving this milestone,” Techsomed CEO and Founder Yossi Abu said in a Sept. 5 announcement.
Key features of the software included 3D visualization of the ablation target, the ability to overlay virtual instruments and estimated ablation regions over medical images, and interactive 3D views of ablation margins and missed volumes.