On the Horizon: Three Imaging Technologies Head Toward the Clinic
In 2013, the National Institute of Biomedical Imaging and Bioengineering (NIBIB) celebrated its tenth year of operating as a focal point for advancing the development of technologies to improve the health of the U.S. population. Bill Gates once said: “We always overestimate the change that will occur in the next two years and underestimate the change that will occur in the next ten.”1 Looking back over the past decade, we have seen a significant change in how radiology is practiced—but what research tools currently being developed might change radiology practice in the next decade?
Shortly before NIBIB was founded, Health Affairs published a physician survey asking about the most important medical innovations of the previous 25 years. Doctors rated MRI and CT scanning as the most significant developments ahead of twenty-nine other innovations including statins, hip replacements and bone marrow transplants.
In 2014, it is estimated that more than 33 million MRI procedures will be performed in the U.S., up 40% from a decade earlier; there were 81 million CT procedures performed, up 52% over the same period. Ultrasound procedures have become equally as ubiquitous. It is clear that the ability to noninvasively see inside the body has revolutionized medicine; these technologies can now aid in early diagnosis as well as enable monitoring of both disease progress and treatment efficacy. There also is a growing demand for imaging procedures to be able to identify functional and molecular characteristics of abnormal pathology that inform treatment, in addition to simply identifying location.
There are many exciting, new imaging techniques that are continually being developed, with NIBIB receiving more than 850 applications in 2013 related to developing new technologies. As Gates once observed, after the discovery and demonstration of a new approach, we see a burst of enthusiasm for investigating this new, potentially game-changing technique. In practice, however, it often takes close to two decades before a technique is ready for wide adoption into clinical practice.
Maintaining momentum through this long translational process of optimizing and validating promising imaging solutions is challenging, and often relies on bringing together multidisciplinary teams that can pursue research questions, develop convincing evidence for safety and efficacy and design products that are attractive to end-users. Below are three areas in which NIBIB is funding technologies that may lead to new clinical procedures within the next decade.
Elastography
Palpation, employed by medical practitioners for millennia, is used to detect differences in the stiffness of tissues. It is considered an effective method for detecting pathologies, but is limited to tissues accessible to a physician’s hands and is qualitative in nature. To overcome these limitations, both ultrasound and magnetic resonance techniques have been developed. Ultrasound techniques have been available for more than two decades and several systems have been approved for use by the FDA in the past six years; however they are still considered experimental by many insurers. Magnetic resonance elastography (MRE) was developed in 1995 and offered improved image reconstruction, assessment of tissue anisotropy, access to organs like the brain that are not accessible by traditional ultrasound approaches and decreased operator dependency. However, long acquisition times are a disadvantage.
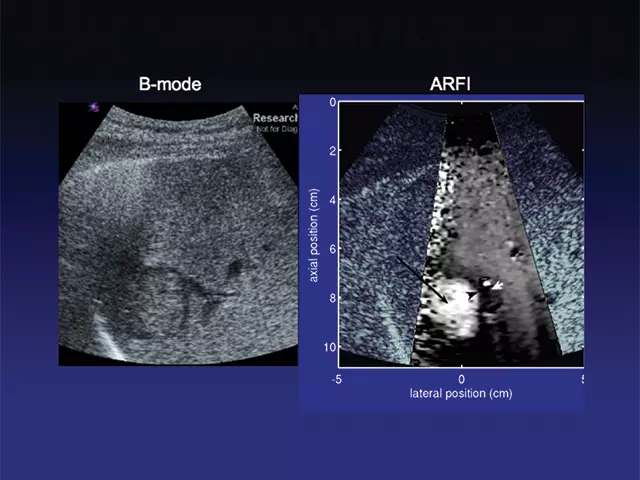
NIBIB has been actively funding research into removing some of the barriers to further clinical translation of ultrasound elastography. Several current projects study acoustic radiation force impulse imaging (ARFI) and shear wave elasticity imaging (SWEI), both of which are expected to be less operator-dependent. ARFI uses focused, high-intensity sound beams to produce “push-pulses” that generate shear waves (secondary waves that extend in a direction perpendicular to the direction of the push pulse) within tissue; and then monitors the tissue response with ultrasonic methods.
The tissue response is related to the stiffness properties and structure of the liver and is displayed as high resolution, qualitative elastographic images of the liver. The speed of the shear waves is proportional to the stiffness of tissue; thus ARFI can also produce quantitative stiffness measurements based on the speed of the shear waves. These measurements can then be used to quantitatively map tissue properties; for example, to classify different stages of liver fibrosis or lesions (Figures 1 and 2).
Other projects use elastography and related methods to classify thyroid nodules, map the myocardium with the potential for improved early detection and localization of ischemia and measure the mechanical properties of tendons and ligaments for quantifying the benefits of physical therapy. The technique has been licensed and adopted for use in new ultrasound imaging systems in Europe, and clinical studies there and in Asia are showing consistently good results. While this tool is not yet available in the U.S., the vendor is currently pursuing FDA approval to market it in the U.S.
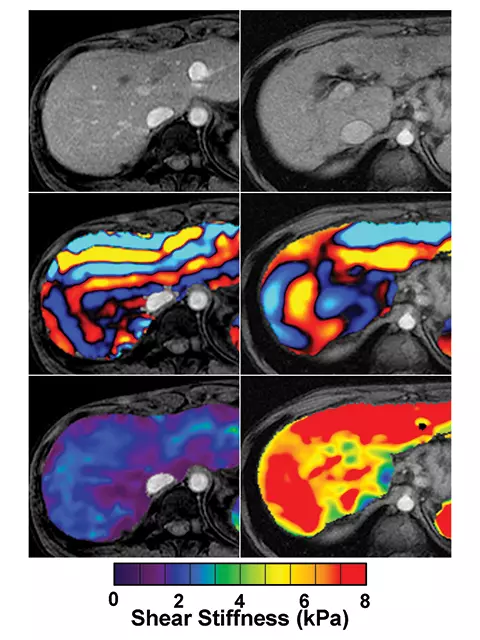
Magnetic resonance elastography (MRE) also is another area of active research. It too relies on shear waves to image tissue stiffness. A vibration source is placed against the abdominal wall and a novel MRI technique captures images of the waves, which are analyzed with an algorithm, developed especially for the MRE process. The algorithm translates the wave images into a quantitative map of tissue stiffness.
MRE is complementary to the ultrasound techniques, and current projects are investigating the use of MRE to quantify the properties of kidneys, pancreas, adrenals, small bowel, uterus and prostate, as well as the liver (Figure 3). Another project is studying animal models of lung inflammation and fibrosis for better characterization of the underlying pathophysiology of interstitial lung diseases. A third project is using MRE to develop poroelastic and viscoelastic models of the brain, with the goal of achieving better characterization and discrimination between different neuropathophysiological diseases, such as Alzheimer’s disease and multiple sclerosis.
Phase-contrast CT
Phase-contrast imaging helped revolutionize light microscopy in the middle of the last century, leading to a Nobel Prize for Frits Zernike in 1953. It has become the standard technique for visualizing cellular structures in live cells because it converts the optical density of biomolecules at boundaries, such as mitochondria (which are hard to see under white light absorption imaging) into intensity variations that can be more easily visualized. The same principles apply in x-ray imaging. Standard x-ray systems are based on measuring the attenuation of a beam through a biological sample in order to determine its structure. By comparing the intensity of the transmitted x-rays with the incident beam pattern, an image of the absorption of the sample at that angle and energy range can be recorded.
X-ray beams also are influenced by variations in density, which changes the phase of the wavefront of the beam. By converting the phase of the transmitted x-rays into variation in intensity, the phase image of the sample can also be read out. The key advantage of phase-contrast CT, sometimes called phase-sensitive imaging (PSI), is that it is much more sensitive to density variations than conventional x-ray absorption CT, giving much better contrast for soft tissue. This sensitivity to density changes can reveal soft tissue features that may have only one thousandth times lower change in absorption. It also potentially can be used to reduce radiation exposures because it does not need a significant absorbed dose in order to generate an image.
With these advantages, why is it not more widely used? Bonse and Hart first demonstrated X-ray phase-contrast imaging fifty years ago, but their technique couldn’t be used with conventional x-ray tubes. In the 1990s, a number of additional techniques were developed that were more simplistic, capable of analyzing small angle scattering or fully quantitative, though still requiring a source with sufficient transverse coherence to work.
Thanks to more recent advances in the nanofabrication of gratings, the x-ray equivalent of the Talbot and Talbot-Lau interferometers were demonstrated a decade ago. It was later proven that these techniques can be used with laboratory x-ray sources rather than the much larger and complex synchrotron sources previously used. The realization of techniques that are compatible with standard sources has reduced one of the significant barriers to the clinical use of phase-sensitive x-ray imaging.
NIBIB funds several projects studying the development and translation of this technology for clinical use. One project explores the optimal imaging geometries for mammography at higher energies and for quantitative imaging. The goal of this project is to improve the detection of soft tissue features in dense breasts while minimizing the radiation.
Another of the technical advances that NIBIB currently funds is the development of new types of gratings with smaller periodicity and higher efficiency. These gratings will be tested in a small-animal scanner where the goal is to achieve 30 micron resolution over a 3 cm region of interest using a lower radiation dose and a faster scan time than a comparable micro-CT system. A third project is developing a high resolution CT system for studying large body joints, with the goal of earlier identification and better characterization of rheumatoid arthritis and osteoarthritis.
CEST
The most common forms of magnetic resonance imaging (MRI) rely on our ability to measure the interaction of bulk water protons in the body with external magnetic and electromagnetic fields. Biological structures and exogenous contrast agents can perturb this proton spectroscopy, enabling the identification and characterization of regions of interest. Due to the lower sensitivity of MRI, high concentrations of contrast agents are required to see a reliable signal for exogenous labelling of specific targets, which has led to some safety concerns.
As an alternative, in 2000 it was suggested that using chemical exchange and cross relaxation of protons residing in other biomolecules would provide a distinct spectroscopic measure. The chemical exchange process dominates if the exchange is fast, and this approach has become known as chemical exchange saturation transfer (CEST). This transfer signal can be detected by optimizing the radiofrequency pulses for the protons of interest and results in a small shift in the frequency from the normal water peak. The intensity of the CEST signal depends on both the concentration of the agent as well as the exchange rate of the protons of interest with water protons, allowing this shift to be tuned through chemistry. Because the shifts can be tuned and measured independently, there is also the potential for multicolor CEST imaging.
Currently CEST also requires high concentrations of contrast agents and high radiofrequency power to see a signal, but it can be used to track compounds containing amine, amide, hydroxy and imino groups, reporters that generate proteins expressing these groups and paramagnetic compounds. There is particular excitement around the use of natural compounds, such as sugars and proteins, which are biodegradable and can interact with different biological pathways.
The flexibility in the nature of the chemical exchange process also has led to the development of imaging protocols and contrast agents for temperature measurement, pH sensing, metabolite detection, nucleic acid binding and labelling of multifunctional nanoparticles. A number of demonstrations using human subjects have been carried out in the past fifteen years, including imaging of urea in the kidney, the use of amide proton transfer to separate edema from tumors and imaging glucosaminoglycans in the knee to identify patella lesions.
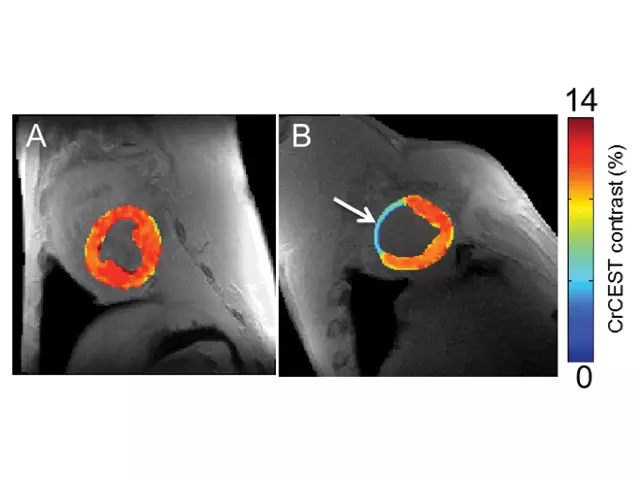
In this area, NIBIB funds several projects. Researchers at Johns Hopkins University are developing CEST reporters for imaging glucose metabolism in brain tumors, measuring zinc release from pancreatic beta cells and monitoring the status of implanted islet and mesenchymal stem cells. Other researchers are developing a wide range of tools including endogenous markers for assessing metabolic injury during acute stroke, markers to distinguish between tumor recurrence and radiation necrosis following radiation therapy, and a variation of CEST called chemical exchange rotation transfer, for studying phosphocreatine and creatine in muscle for better characterization of peripheral artery and heart disease (Figure 4).
Conclusion
It is always difficult to predict which technologies will have a significant impact on future clinical practice because short-term promise does not necessarily translate to high impact, long term. That is why we believe it is important to support a diverse range of basic and translational research projects to develop early-stage technologies. It also is difficult to predict which technologies will be readily adopted by the medical imaging community, but we believe the trend is toward better molecular and metabolic characterization in vivo, to minimize the need for further intervention.
Technology alone cannot achieve the change, because it requires end-users, researchers and industry to come together to realize practical solutions that inform treatment choices. Government agencies such as NIBIB play a critical role in bringing these communities together, supporting high risk projects and helping to develop the evidence that will lead to regulatory approval and reimbursement. There is a strong pipeline of exciting technologies that are moving towards the clinic and a lot of excitement about the change that could be realized over the next decade.
Richard Conroy, PhD, is director, Division of Applied Science & Technology, National Institute of Biomedical Imaging and Bioengineering.
Reference
1. Gates B, Rinearson P, Myhrvold N. The Road Ahead. New York, NY: Viking Penguin; 1996.