Breast density notification laws blanket 90% of U.S. women, yet still no national reporting standard is at hand. Why is that?
Dense breast tissue has been a major topic of discussion in breast imaging for the past decade and has become a driver for the adoption of new technologies. Patient education efforts and many states adopting breast density inform laws have opened more technical discussions with patients about clinical risks and issues with reading their exams.
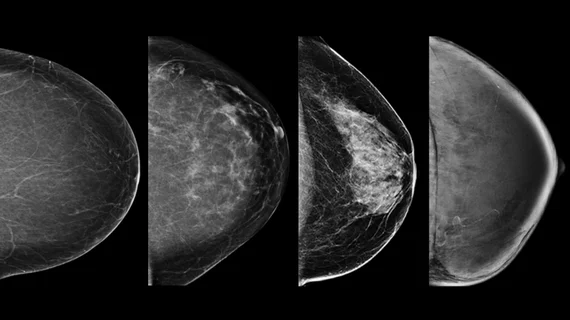
Dense breast tissue is itself a risk factor for breast cancer. The thicker tissue appears white on X-ray mammograms, the same as breast cancers, so it can be difficult to find smaller cancers masked by areas of dense tissue.
Having dense breast tissue is a risk factor many women are unaware they have. Up until about a decade ago, there were no laws requiring radiologists or healthcare organizations to inform patients they are at increased risk of cancer or that they could possibly be subject to having cancers missed on their annual mammograms due to increased breast density. There are now close to 40 states with laws on the books requiring physicians to notify patients with dense breasts that they have the trait and to inform them on what this means for their care.
Radiology Business asked two experts to share their thoughts on where things stand in terms of addressing breast fibroglandular tissue density on the fronts of legislation and new technology.
• Wendie A. Berg, MD, PhD, FACR, is a professor of radiology at Magee-Women’s Hospital of University of Pittsburgh Medical Center (UPMC), University of Pittsburgh School of Medicine, and is well known for her role as study chair and principle investigator of the ACRIN 6666 trial that exams, screening breast ultrasound and MRI in high-risk women. She also serves as the chief scientific advisor to the patient advocacy group www.DenseBreast-info.org.
• JoAnn Pushkin, executive director of DenseBreast-info Inc., is a breast cancer survivor whose breast cancer went undetected for several years because of dense breast tissue. She was key in pushing for the passage of New York’s breast density patient inform law.
Radiology Business: Where are things at with breast density inform laws and what areas should people be watching?
JoAnn Pushkin: Currently, 38 states and the District of Columbia (DC) have active density inform laws, encompassing over 90% of American women.
Unfortunately, the laws vary widely in terms of information they provide. Six state laws (Connecticut, Louisiana, Maryland, Missouri, New Jersey and Texas) only require that a woman be provided general information about breast density without providing specific information about a patient’s own breast density.
A single national reporting standard is overdue, and it is not clear when the FDA will establish a rule to standardize state reporting requirements. Once there is a national standard, individual state law review will still be necessary, as state laws that are at least as stringent as what is required on the federal level can, generally, opt to keep their current inform language.
I continue to be contacted by women in the remaining states who are interested in “inform” advocacy and there may be state “inform” law introductions in upcoming state legislative sessions. However, the bulk of recent bill introductions are related to insurance coverage for supplemental screening and/or diagnostic breast imaging. Unfortunately, even if a state enacts an insurance law to cover additional screening to any extent, there are insurance policies (e.g., self-funded plans, federal plans, out-of-state plans) that are exempt from state insurance laws.
DenseBreast-info.org features a state-by-state analysis of inform and insurance laws, here.
Radiology Business: Are there government policies or reimbursement issues that still need to be dealt with?
JoAnn Pushkin: Due to the variety of insurance plans (e.g., private, state, or federal plans), overlayed with variety of inconsistent state insurance laws, it can be a challenge for a patient to know, before receiving a bill, to what extent her breast imaging costs will be covered. And, unless specifically addressed in an insurance law, even if a test is “covered,” copayments and deductibles may not be. The cost to the patient can be high. This uncertainty can cause women to forgo suggested supplemental breast imaging such as screening MRI. Everyone seems to have a horror story of a friend who thought she was covered but then got hit with a hefty bill and spent hours on the phone trying to figure out why.
Questions about insurance coverage or appeals are the most frequent questions submitted to the DenseBreast-info.org “Contact Us” page.
Radiology Business: How is breast density influencing new technology adoption?
Wendie Berg: The ECOG-ACRIN 1141 study of abbreviated MRI in women with dense breasts showed a strong benefit to MRI after tomosynthesis (adding 10 cancers per 1,000 women screened in a year).
With the important results of the DENSE trial of screening MRI in the Netherlands, there is now a European Society of Breast Imaging guideline recommending screening MRI every two to four years for women aged 50 to 70 who have extremely dense breasts. In women unable to have an MRI, ultrasound can be considered.
The National Comprehensive Cancer Network guidelines suggest contrast-enhanced mammography (CEM) or ultrasound in women who qualify for screening MRI but who are unable to have it. Fourteen states, including D.C., now have insurance laws requiring coverage for screening ultrasound and MRI, and two additional states require coverage for ultrasound only.
CEM appears to perform similar to abbreviated MRI. Some centers offer molecular breast imaging as an alternative. There is a great need for more widespread access to contrast-enhanced or functional breast imaging as these are much more effective at finding biologically important disease when it has not yet spread to lymph nodes and is easily treated.
Find more information on cancer detection by screening method, here.
Radiology Business: What is the role of artificial intelligence (AI) in breast imaging in regards to dense breasts?
Wendie Berg: AI can be used to mark areas of concern on a mammogram, ultrasound or MRI. The radiologist could then pay special attention to those areas. Such tools perform at about the same level as a radiologist who specializes in breast imaging, but these tools are not in widespread use at this time. Another use for AI is to “triage” mammograms so that the “easier” ones (most likely normal exams) can be read quickly, and the “harder” ones that are more likely to show cancer could get extra attention.
Perhaps most importantly, AI is showing great promise in predicting which women will develop breast cancer just by looking at the current mammogram. Breast density is a risk factor. Women with denser breasts are both at higher risk of developing cancer and of having cancer masked on the mammogram.
The complexity of the mammogram also factors into risk, with more nodular breast tissue associated with greater risk. It appears that AI is better at identifying who will develop breast cancer in the next five years than are the various risk models or even genetic test results. Using this information could help us more accurately and more easily identify which women should have additional screening (especially with MRI) beyond a mammogram (or instead of it).