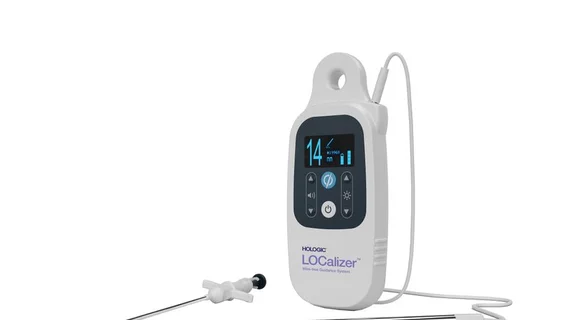
Wireless breast lesion localization device receives CE mark
Hologic has received CE certification for its LOCalizer wireless radiofrequency identification breast lesion localization system.
Up to 30 days before surgery, clinicians can implant Hologic’s LOCalizer tag inside a patient’s breast and then use the portable, handheld reader to pinpoint the exact location of breast lesions before breast tissue removal surgery.
The system is intended to replace traditional methods for localizing breast lesions, which are often wire-guided, according to the company.
“We look forward to showcasing the new LOCalizer system at ECR as we continue to broaden our offerings to make a positive impact on breast health at every step of the patient journey – from screening to pathology,” Jan Verstreken, Hologic's regional president for EMEA & Canada, said in a prepared statement. “This thoughtful expansion is rooted in our commitment to developing new and innovative solutions clinically proven to improve cancer detection, patient satisfaction and facility workflow, while reducing costs associated with unnecessary callbacks.”