Canon Medical Components U.S.A., Inc. announces launch of new products in the US to be on display at RSNA 2022
Canon Medical Components U.S.A., a subsidiary of Canon, today announced new products line-up at the Radiology Society of North America (RSNA) conference and annual meeting, held Nov. 27 through Nov. 30 at the McCormick Place Convention Center in Chicago, Illinois (Booth # 8127, North Hall Level 3). RSNA 2022 is a global radiology forum where the power of imaging, education and collaboration come to life. Canon Medical Components U.S.A., Inc. is displaying a number of innovative products, some that are now available for the first time in the United States.
The CXDI-Pro series of wireless digital radiography (DR1) devices
The Canon CXDI-Pro wireless digital radiography systems are designed to support the demands of medical imaging departments for cost-effective solutions without compromise. These new wireless detectors provide features that optimize workflow and offer the high quality and reliability that you have come to expect from Canon.
The CXDI-Pro series employs the lightweight, CXDI-703C Wireless sensor unit, which weighs approximately 2.9 kg. The new series, designed to reduce the physical burden on patients, inherits the same IP55 standard3 compliant dust and water resistance of predecessor models allowing for frequent cleaning of the imaging portion and protecting the device in operating rooms and other environments where stray droplets are common. The device design also has fewer connection seams and visible screw heads in surfaces that come in contact with patients.
The CXDI-Elite series of wireless digital radiography (DR) devices
The CXDI-Elite series has high sensitivity, high image quality, and an ultra-lightweight, ergonomic design for ease of handling long battery life and AED4 function. This makes the CXDI-Elite the ideal digital radiography detector for mobile applications or any general x-ray need. The unique functions, Intelligent NR and Built-in AEC5 assistance expand the digital radiography possibilities.
New "Built-in AEC Assistance" technology for digital radiography (DR)
The CXDI-Elite series is Canon's first digital X-ray imaging system to utilize Built-in AEC Assistance6 technology designed for general X-ray imaging. With this technology, the device's X-ray image sensor allows for automatically terminated exposures without the use of an additional receptor (ion chamber, solid state paddle, etc.) This function works via both wireless and wired communication, which enables the optimization of X-ray dose without an external AEC sensor, even in free-position imaging such as bedside.7
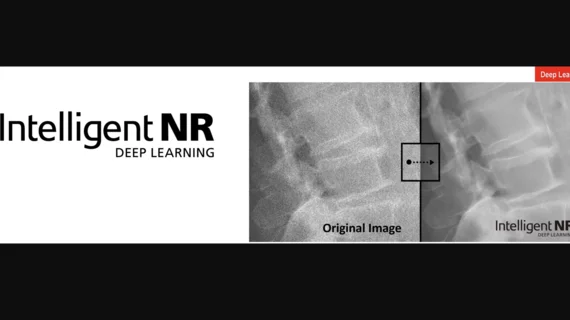
New "Intelligent NR" technology for control software8 for CXDI series of digital radiography (DR) devices
This Intelligent NR is new technology for DR control software that utilizes AI technology to reduce digital radiography image noise. Intelligent NR is based on Canon's proprietary AI technology that utilizes deep learning and has been trained using approximately 3,000 X-ray images obtained over the course of the company's long history of developing the CXDI series of clinical imaging. Intelligent NR will provide radiologists with high-quality diagnostic images containing significantly less grainy noise with no noticeable loss of detail. This should make improved diagnosis possible for their patients. Intelligent NR makes it possible to reduce the noise content without losing the fine details of the anatomy, even in low dose regions. Intelligent NR results in an optimal diagnostic environment, especially for infants and pediatric patients where dose is a prevailing concern. But the image improvement will benefit all patients and exam types by providing superior images even in inherently noisy conditions such as the dense anatomy of the abdomen. This new technology has the potential to support better-quality diagnoses on the front line of medical treatment and make possible high-quality imaging with reduces X-ray dosages for patients.
Availability
These new products are now available through Canon Medical Components U.S.A., Inc.
For more information and the full list of product specifications, visit https://mcu.canon
About Canon Medical Components U.S.A., Inc.
As part of Canon's global strategy to expand its medical components business, Canon Medical Components U.S.A., Inc., has been established as a new subsidiary of Canon Inc. in the U.S. Canon Medical Components U.S.A., Inc. will focus on working directly with medical equipment manufacturers. As of July 1, 2020, the digital radiography components business of Canon U S.A., Inc. has been transferred to Canon Medical Components U.S.A., Inc. Visit https://mcu.canon.