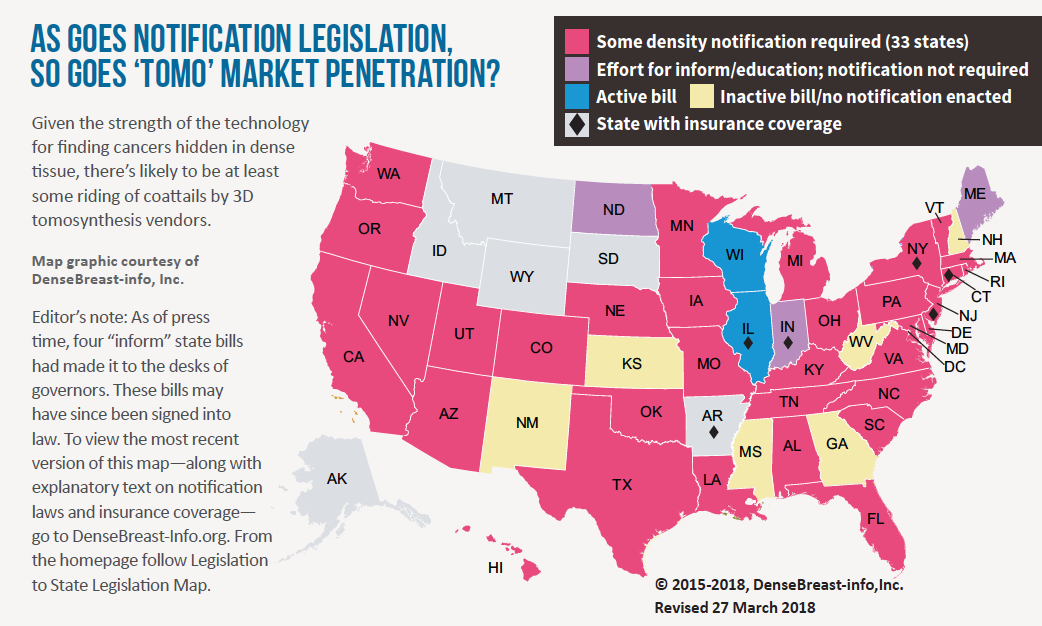
There’s no stopping 3D mammography now, but can it sustain this pace?
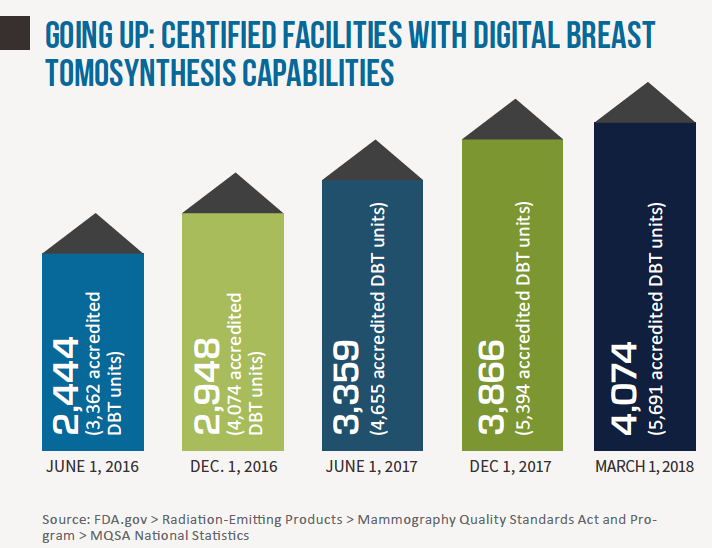
Seven years after the FDA approved the first tomosynthesis device for breast cancer screening, adoption rates for digital breast tomosynthesis (DBT) remain on an upswing. Earlier this year the agency reported a nearly 30 percent increase of certified mammography facilities offering DBT—aka “3D mammography,” aka “tomo”—over just the past year (from 3,178 facilities in March 2017 to 4,074 in March 2018). Meanwhile the American College of Radiology estimates that about 30 percent of mammography units installed in U.S. hospitals and imaging facilities now have 3D capabilities. Precise comparison percentages are hard to come by, but that ratio represents a marked increase from a few short years ago.
The rise of DBT is no surprise. Many perceive tomosynthesis as a game-changer for radiology, and even those who might not characterize it as a must-have mammography modality acknowledge that its pros outweigh its cons.
Jay Baker, MD, professor of radiology and chief of the breast imaging division at Duke University Medical Center in Durham, N.C., and vice president of the Society of Breast Imaging, calls DBT “the rare win-win” in medical imaging, in large part because it “markedly reduces” the likelihood of overlooking cancer that may be concealed by overlapping tissue while also mitigating against unnecessarily calling patients back for additional imaging due to questionable findings.
In DBT screenings, multiple limited-angle projections are acquired from the standard craniocaudal and medial lateral oblique mammographic screening positions under compression. The images are then reconstructed by algorithm into slices for z directional viewing of mammographic breast tissue.
“At least a dozen studies all confirm that tomosynthesis helps us identify about 25 percent more breast cancer than we see on routine 2D mammograms,” Baker tells RBJ. “Combined, these studies were performed on more than half a million patients, so there really is no doubt that tomosynthesis is better in that regard. In those same studies of hundreds of thousands of women, radiologists reduced their recall rates by about 15 percent to 35 percent. That’s huge”—and can translate to significant reductions in unnecessary biopsies.
Evidence-based Advantages
Stephen Rose, MD, concurs. “Our callback rates have decreased by nearly 40 percent as a result of implementing 3D screening, while our positive predictive validity, which previously was at 20 percent to 25 percent, is now 40 percent,” reports Rose, who is founder and president of Houston-based Rose Imaging Specialists and CMO of multi-state, 44-site Solis Mammography. “With 2D, there is a need to sacrifice specificity to increase sensitivity, but with 3D and the opportunity to better evaluate small slices, this is not the case.”
“When mammography went digital, I didn’t get excited. But with 3D, there is that excitement,” he adds, “especially because of ongoing improvements to technology that do and will make it a winner.”
And the pro-DBT evidence keeps coming. At RSNA 2017, Maryam Etesami, MD, and colleagues at Yale presented new retrospective research analyzing more than 44,000 mammograms, 64 percent of which included DBT versus 36 percent that were 2D digital mammography alone. The researchers found DBT screening detected more small cancers with fewer positive axillary lymph nodes than did 2D digital mammography. Further, axillary lymph node metastasis in invasive cancers was reduced to about half with DBT screening (14.7 percent) compared to 2D-only screening (30.9 percent).
DBT screening “may improve breast cancer early detection compared to digital mammography, which may lead to less systemic treatment and improved clinical outcomes,” the team concluded.
Elizabeth Morris, MD, chief of breast imaging at Memorial Sloan Kettering Cancer Center in New York, sees a slightly different—albeit still positive—picture.
“Tomosynthesis with 2D is better than 2D mammography, that is for certain,” she says. “However, there’s still concern about missing cancers in extra-dense breasts, so it’s difficult to say it’s game-changing at this point. In my opinion, the advantage of DBT is not that it picks up more cancer but that it decreases recall rates by giving us a better look at the breast.”
Mileage May Vary
No matter where clinicians perceive DBT to be on the game-changing/win-win scale, few deny that it has its limitations and challenges. On the clinical side, for starters, there are patients for whom tomosynthesis is a beneficial yet not entirely adequate screening modality.
Dana Smetherman, MD, MPH, of Ochsner Health System in New Orleans and vice chair of the ACR’s commission on breast imaging, places into this category women whose lifetime risk of developing breast cancer is 20 percent or greater based on such factors as a family history of the disease, BRCA1 or BRCA2 gene mutation and/or high-risk lesions (e.g., lesions in situ).
For Smetherman, women who underwent irradiation of the chest at 10 to 30 years of age fall under this same umbrella.
“For most patients, DBT is an excellent screening option given the decreased recall rates as well as reduced false positives and increased cancer detection rates,” Smetherman says. “But when it comes to the high-risk population, a more comprehensive approach is preferable. We [at Ochsner] follow the ACR’s recommendation for patients in the ‘20 percent plus,’ group.” This calls for alternating between breast MRI and a combination of 2D mammography and DBT at six-month intervals.
According to Baker, DBT screenings don’t always identify breast cancers in the 10 percent of women whose breast tissue is extremely dense. Additionally, he observes, there are some instances in which tomosynthesis is “not the best choice” for further evaluation of breasts in which an abnormality has been identified. He cites as an example microcalcifications seen on screening mammograms. These, he believes, are much better evaluated with 2D spot-compression magnification mammograms.
The latter, Baker explains, offer greater resolution than tomosynthesis, allowing radiologists to do a more effective job determining the morphology, number and distribution of calcifications. From this 2D starting point, the radiologist can better identify calcifications that may signify the presence of precancerous cells or breast cancer.
Daunting Radiation Doses
Another frequently cited clinical drawback of DBT: It’s usually performed in tandem with—rather than in place of—2D imaging for routine breast cancer screenings. For this reason, patients undergoing combination 2D/DBT studies receive twice the amount of radiation to which they would otherwise be exposed. While such a dosage does not exceed the limit mandated by the FDA, the increased exposure remains a cause for concern among some clinicians.
Many practices have moved forward with a workaround to the dosing problem, substituting synthetic mammography (SM) solutions that allow them to create mammograms from projection images obtained during DBT exams. Instead of using the data to reconstruct tomosynthesis slices, as occurs with DBT, these solutions leverage a computer algorithm to reconstruct what Baker says “appear to be regular 2D mammograms,” without exposing patients to another dose of radiation.
In addition to reduced radiation dosage, proponents of SM say it yields ancillary benefits, including shorter image acquisition time compared with traditional combination 2D/DBT studies and increased ability to identify calcifications, spiculated margins and architectural distortion.
“A radiologist who is familiar with synthetic mammograms can tell a synthetic mammogram apart from its [2D counterpart], but sometimes you have to look closely because they are very, very similar,” Baker observes. “In fact, synthetic mammograms provide us with essentially all the information we need that may not be visible in the tomosynthesis slices.”
Certain study findings appear to support Baker’s assertion. In research published online ahead of print last September in Clinical Breast Cancer, Nehmat Houssami, MBBS, MPH, PhD, of the University of Sydney concludes that comparative population screenings indicate little difference between cancer detection rates achievable utilizing integrated 2D/3D mammography units (range, 5.45 to 8.5 per 1,000 screens) and units that generate 2D synthetic images of the breast from images acquired via DBT (range, 5.03 to 8.8 per 1,000 screens). This was so, he writes, despite “relatively heterogeneous” recall rates across all included studies.
The Synthetic Substitute
A study published online in Radiology last December presents similar findings. The authors evaluated 16,000 asymptomatic women ages 50 to 69, all of whom underwent DBT and synthetic 2D mammography exams through the Breast Cancer Screening Program in Italy between April 2015 and March 2016. They then compared data pertaining to these patients with data from 2D mammography exams performed on more than 14,000 women who had been screened through the same program the previous year. Cancer detection rates with DBT plus synthetic 2D imaging turned out to be significantly higher than cancer detection rates with traditional 2D imaging—9.3 per 1,000 exams versus 5.41 per 1,000 exams.
“There was evidence of improved cancer detection with DBT plus synthetic 2D imaging across age groups, with significant differences for women older than 55 years, further highlighting the effectiveness of DBT in terms of cancer detection for women in this age group,” write lead author Francesca Caumo, MD, of Ospedale di Marzana in Verona and colleagues. “DBT plus synthetic 2D imaging also significantly improved cancer detection in all density-stratified analyses.”
But even clinicians who favor the use of SM acknowledge that it, too, has its drawbacks. Caumo et al represent a case in point. They conclude in their study that a screening protocol in which DBT is employed to complement SM is safe, effective and offers the benefit of decreased radiation dosage. However, they also point to SM’s artifacts and “weaknesses” and, by extension, the “weaknesses” of SM and DBT used together.
The former encompass “blurring subcutaneous tissue, loss of resolution in the axilla on mediolateral oblique views, pseudo-calcifications and decreased resolution near foreign bodies, e.g., biopsy markers”; the latter, the “potential for false positives due to pseudocalcifications and the difficulty in assessing for motion artifact.”
Similarly, some radiologists note reduced sensitivity for identifying calcifications when screening is performed utilizing a combination of SM and DBT. In such cases, they hold, the clinical benefits of finding and addressing calcifications may outweigh the risk inherent in exposure to the additional radiation needed when DBT is paired with traditional 2D mammography.
Wending Workflows
Extended interpretation times are said to constitute an equally significant challenge when employing DBT in breast cancer screening. By most estimates, DBT studies comprise a total of 200 to 400 images, depending on breast size and equipment used. Interpreting these images reportedly takes 1.5 times to as much as 2.5 times longer than reading only the two images per breast—one top to bottom and one side to side—captured during the 2D component of each exam.
In the aforementioned Italian study, the mean number of screening procedures interpreted was markedly lower for DBT/2D mammography procedures than for 2D mammography procedures alone, totaling 38.5 and 60 examinations, respectively.
Commenting on the commonness of longer lag times for DBT results, Morris of Memorial Sloan Kettering notes the need for flexibility among breast-imaging teams.
“Adjustments to workflow and the addition of staff may be necessary to accommodate the extra time needed with so many images added to the mix,” Morris says. However, she adds that, in her experience, clinicians’ level of confidence in the findings of DBT examinations increases as they become accustomed to the ins and outs of interpreting 3D images, in turn making the process somewhat more efficient.
Reductions in the number of recalls also help to “balance things out a bit,” she adds, although there is still “no getting away from the fact that DBT requires a big time commitment and involves a complicated workflow.”
Emily Conant, MD, division chief of breast imaging at Penn Medicine in Philadelphia, suggests that making a gradual transition to DBT could minimize workflow complications over the long run. She recommends starting with screening cases rather than attempting to simultaneously harness DBT for initial screenings and follow-up exams. Limiting screenings to first-time mammography patients at the outset, and until read times are on a more even keel, can also prove helpful, Conant says.
An Exorbitant Entry Price for Some
Perhaps unsurprisingly, the roadblocks encountered on the road to widespread DBT adoption extend to the technology itself, as tomosynthesis units carry a steep price tag. According to various vendors in the space, even models with few, if any, bells and whistles run about $400,000 to $430,000 apiece. Traditional 2D mammography equipment necessitates a much lower outlay—approximately $80,000 to $100,000 per unit.
Some sources say it is possible for practices whose budgets do not currently support an investment in tomosynthesis for the purpose of breast cancer screening (and follow-up diagnostics) to postpone migrating to the modality until circumstances allow—or to eschew it permanently. “This is particularly true for those that have in place a quality 2D imaging [equipment component] that supports a high standard of patient care and can supplement 2D imaging with ultrasound,” Morris states.
Others favor a slightly different approach. In a study published in the Nov. 2015 edition of the American Journal of Roentgenology, Conant and colleagues advocate a solution for financial constraints to DBT implementation wherein practices identify patient populations that will benefit the most from tomosynthesis and then tap the technology to perform screenings only those patients.
According to the study, the recall rate for baseline screening is “ especially pronounced” in women under the age of 50. “If resources are limited,” the authors write, clinicians might consider focusing their tomosynthesis efforts on “women younger than 50 years who are undergoing baseline screening or do not have prior available mammograms,” as they may “benefit more from digital breast tomosynthesis than from digital mammography alone.”
Unstoppable Momentum?
As for other technology-related constraints, there are issues around image size and interpretation. For starters, typical tomosynthesis studies average approximately 450 megabytes in size, but can be as large as 3 gigabytes, leading to potential storage constraints.
In deploying DBT technology, Smetherman quickly saw the need to acquire additional image storage space to accommodate the larger images, as well as to prevent the system from “locking up” and impeding the workflow.
“Because there are so many images to store, it’s important to really consider what you are going to keep and what you are going to throw away,” Morris notes. She adds that, in order to accommodate additional, larger images, her practice discards raw data and keeps only 3D images.
Both Smetherman and Morris point to possible difficulties in interpreting 3D images if PACS conditions are not right. Morris obtained a separate workstation to enable 3D to 3D comparisons, and Smetherman, a separate PACS with a 3D image hanging protocol.
In Texas, Stephen Rose is sanguine. “Breast tomosynthesis has many positives—too many not to recognize,” he underscores. “As we’ve already seen, yes, there are challenges—but those challenges will continue to be overcome.”