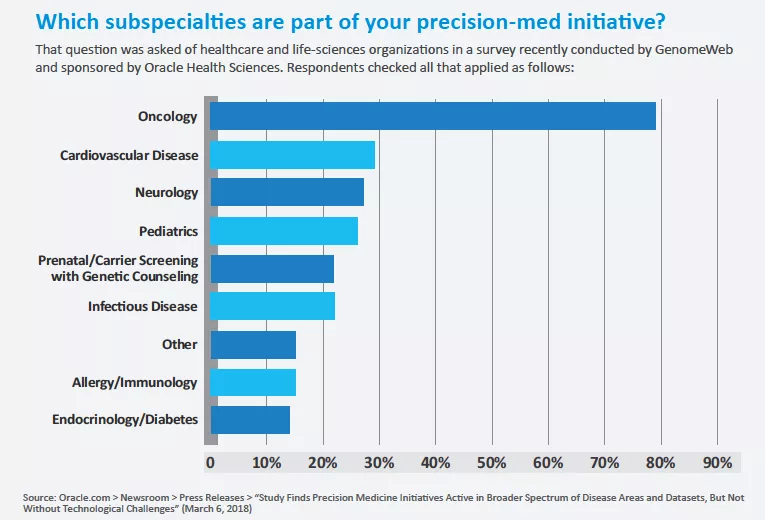
Precision medicine saves a seat for imaging expertise
Picture this. A woman with breast cancer and a BRCA gene mutation is being evaluated to assess the appropriateness of a particular drug therapy. Instead of the usual tissue biopsy—or in addition thereto—the lead oncologist orders molecular imaging with a novel radiotracer. BRCA-associated malignancies tend to have telltale imaging features on PET scans, so the imaging will help guide the care: Will this particular cancer respond to this particular drug therapy or not?
This increasingly common scenario exemplifies the developing role of imaging in precision medicine. There’s some debate over the interchangeability of this term with “personalized” medicine, but the National Research Council prefers the former and broadly defines it as treatments and preventions focused on “identifying which approaches will be effective for which patients based on genetic, environmental and lifestyle factors.”
The precision approach, in other words, separates patients likely to benefit by a given intervention from those who have the same disease but are better off avoiding that intervention’s expenses and side effects. Or, simpler still: the precise treatment for the right patient at the right time.
Radiologists working in the new world of genetics-based medicine are finding an emerging opportunity to take a prominent seat at the precision-medicine table. The only question is how close to the head of the table they’ll sit as the technology matures. The answer to that question is coming into clearer focus day by day.
“While there are some challenges, the word ‘pivotal’ best defines the part imaging is evolving to and will play in precision medicine,” says radiologist Supriya Gupta, MD, of Rush University Medical Center in Chicago. Gupta co-authored a review article published in Academic Radiology showing that imaging is already playing a critical role in numerous aspects of precision medicine, including screening, early diagnosis, treatment guidance, efficacy evaluation and assessment of likelihood of disease recurrence.
Further, Giardino, Gupta and colleagues assert, imaging will play an increasingly important role in applying precision medicine to non-cancerous medical conditions. “Imaging phenotypes, including imaging-based classifications and scoring systems,” they write, “can help to classify patients with similar disease manifestations into distinct subgroups, improve clinical trial designs, better evaluate treatment response and improve patient outcomes.”
Regardless of the illness, the experts agree, the growth of precision medicine will challenge radiologists to think of themselves as less in the business of disease diagnosis and more in the business of treatment planning.
Metabolic-molecular insights
New applications for imaging in precision medicine continue to come to light. James Thrall, MD, chairman emeritus of the radiology department at Massachusetts General Hospital, notes that precision medicine harnesses phenotyping to separate “like” patients into groups, according to their particular prognosis. He describes a system developed by a colleague for scoring patients who had suffered an intracerebral hemorrhage based on the number of contrast spots found on their CT angiogram. Patients are divided into risk categories in keeping with levels of bleeding indicated by the contrast spots, Thrall says, adding that the system is being tested in several pilot programs.
Thrall adds that imaging’s most significant impact on precision medicine may unfold in molecular imaging. He cites the example of utilizing FDG PET scans in creating phenotypes to assess Hodgkin’s lymphoma patients after several rounds of chemotherapy. In some patients, the procedure shows no abnormal uptake of the tracer, indicating a normal life expectancy; in others, the continued presence of abnormal uptake signals a significantly lower life expectancy.
“Those phenotypes provide us with much more accurate information than would be possible using the International Prognosis Scoring System,” Thrall tells RBJ.
In a study published in the January 2017 edition of PET Clinics, Katherine Zukotynski, MD, of McMaster University in Ontario and colleagues show how PET contributes to precision medicine by “interrogating tumor heterogeneity throughout the body.” Accordingly, Zukotynski and co-authors write, it is “extremely helpful not only to distinguish resectable from non-resectable disease but … to suggest the most appropriate therapy at a metabolic-molecular level.” They stress that this application makes PET imaging a key component in formulating a “subsequent strategy for patients with lung cancer, including monitoring of therapy response, detection of recurrence and prediction of patient outcomes.”
Meanwhile, Maryellen Giger, PhD, a radiology professor and vice-chair of radiological research at the University of Chicago, notes that effective diagnosis and treatment of disease increasingly rely on the integration of information from multiple patient tests involving clinical, molecular, imaging and genomic data—in other words, various “-omics.” Radiomics “has been defined as the conversion of images to minable data,” Giger writes in a March 2018 JACR article on machine learning in medical imaging. “Obtaining radiomic data may involve computer segmentation of a tumor from its background followed by computer extraction of various tumor features. The ultimate benefit of quantitative radiomics is to yield predictive image-based phenotypes of disease for precision medicine or [to] yield quantitative image-based phenotypes for data mining with other -omics for discovery (i.e., imaging genomics).”
Cautious interpretations
With opportunities for radiology to help propel precision medicine forward come a wealth of challenges, some of which have yet to be addressed. A few such challenges center on data. For example, Gupta notes that there now exists an enormous volume of Big Data pertaining to patients, including data that can be extracted not only from their genomics and radiomics but also their epigenomics, transcriptomics, proteomics and metabolomics—googling encouraged here—as well as from their EHRs and other sources. Individual data elements must be integrated, interpreted and analyzed, with true and relevant data separated from not-so-true and/or irrelevant data in order to decrease the potential for erroneous interpretation. However, Gupta says, integrating, interpreting, analyzing and separating data will be difficult unless complex disease-based models are developed and significant investments in bioinformatics and biostatistics have been made.
Yet another challenge for radiology and its role in precision medicine pertains to the interpretation of radiogenomics. In the 2017 Academic Radiology report, Gupta et al. point out that attaining the objectives of precision medicine necessitates using radiogenomic data to classify patients into precise subgroups, basing such determinations on individual patients’ disease phenotypes (including imaging features) and genotypes. However, the authors deem the interpretation process less straightforward than it appears.
For example, they underscore that “not all genetic mutations are directly related to cancer; some of the changes may be secondary to germline mutations. Therefore, a ‘tumor-only’ sequencing approach may be misleading.” They cite a study in the July 2015 issue of Science Translational Medicine concluding that personalizing therapy based solely on a tumor-only sequencing approach may have led clinicians to make erroneous treatment decisions about nearly 50 percent of patients. Performing matched normal DNA sequencing, coupled with tumor sequencing analysis for precise identification and interpretation of somatic and germline alterations, would have been a far preferable option, the study indicates.
“Given these challenges within genomics, the interpretation of radiogenomics data requires caution,” the authors write. They suggest that well-designed prospective trials involving sizeable groups of patients and large public databases, such as the Cancer Imaging Archive and the Cancer Genome Atlas, may help to clarify correlations between imaging phenotypes and the genomic characteristics of different cancers. This, in turn, would allow radiogenomics to play a more pivotal role in precision medicine.
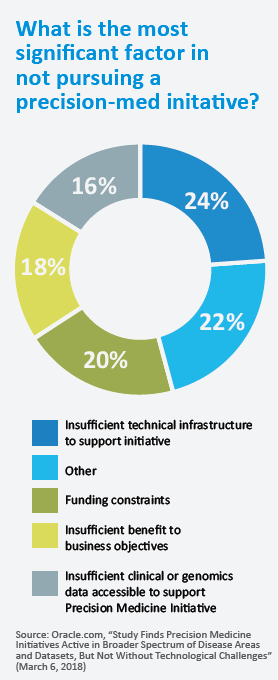 The AI ingredient
The AI ingredient
Radiomics, while considered by many to have great potential to propel precision medicine forward, also poses challenges. A majority of radiomics studies, sources say, are small and have not been sufficiently validated. Most have reported correlation while leaving causation between imaging biomarkers and patient outcomes out of the equation.
“Beyond even that, standardization in radiomics—or really, its absence—is an issue,” because it interferes with the reproducibility of results, Gupta says. She deems the standardization of techniques across cadres of facilities where radiomics studies are underway a key means of remedying this situation. Additionally, Gupta advocates the development of appropriate quality assurance programs to improve the reliability of newer techniques and biomarkers.
Then there are obstacles linked to artificial intelligence (AI) applications, which loom large in the future of precision medicine. Thrall notes that, while these applications enable previously unavailable information to be extracted from images—thereby opening doors for radiology to make viable contributions to the practice of precision medicine—they must be trained in order to be of value in a precision medicine scenario.
Institutions may balk before sharing their image data, he says, which may make it difficult to put together a dataset large enough to enable proper AI applications training.
The AI wrinkles don’t end there. The tolerance of utilizing AI programs in imaging between varied patient populations has yet to be investigated, and the process of defining normal versus abnormal continuously variable biologic data remains difficult. “In any test or measurement, results for a certain percentage of patients will be [deemed] abnormal, when they really are normal,” Thrall observes. “This is because normal ranges are set as a certain number of standard deviations from the mean of a supposedly ‘normal’ patient population. It can be difficult to define nominal criteria for normal versus abnormal in some instances, like setting limits for organ sizes.”
Treatment in front
As the challenges of incorporating imaging into a precision medicine scenario are met, it will be easier for imaging to earn a seat at the precision medicine table. But radiologists can and should do more to secure and retain their position there.
At the most basic level, Thrall says, this means “changing the conversation” by ceasing to focus on and talk about signs and rank, instead couching discussions with medical professionals around imaging markers and phenotypes. It will also entail overcoming inhibitions and misconceptions that surround the use of AI and deep learning (DL), and instead applying them to the analysis of images and data related to images to demonstrate the value of imaging in a precision medicine scenario. “For example, through AI and DL, we can extract information from images that isn’t visible to the human eye—such as the texture, edge [characteristics] and growth rate of a tumor,” Thrall explains. “We need to communicate the advantages of this ability, and we need to keep sharing our findings and publishing our work.” The more radiologists do so, the more clearly others will understand the pivotal role imaging plays in precision medicine, he says.
Daniel Sullivan, MD, emeritus radiology professor at Duke, believes radiology will have a far better chance of finding a seat at the precision medicine table now and going forward if radiologists alter their focus from diagnostics to the optimal therapy for any given patient, producing radiology reports that specify the implications of selecting that particular therapy. Such a change, he says, is essential because the precision medicine model calls for an emphasis on therapy and treatment rather than on identifying what is wrong with patients.
“Radiologists have been in a diagnostic mindset for years and years,” Sullivan says. “However, in the era of precision medicine, they need to think far less about that and more about how imaging information affects the choice of treatment.” Diagnostics takes a back seat to treatment, according to Sullivan, when one understands that no therapy can be considered precise unless validated biomarkers are present to indicate the likelihood of a particular patient’s response and to distinguish patients who are responding to a therapy from those who are not.
Growing into a new role
Thrall concurs, adding that, as imaging exams can be executed repeatedly, assessing patients’ response to prescribed therapy via multiple consecutive studies instead of just one is part of the therapeutic mindset needed for radiologists to secure and sustain a key role in precision medicine. He cites the example of a patient with neurofibromatosis. An initial imaging examination, he says, can determine the full extent of the burden placed on the body by the condition, with the results of that study giving the referring physician an idea as to an appropriate course of therapy. However, only through follow-up studies can a determination be made as to whether the lesions are responding to therapy and, if so, to what degree.
In tandem with heightened emphasis on diagnostics, radiologists must be willing to find ways to assuage concern among referring clinicians that imaging is not an adequate means of monitoring patients’ response to therapy and potential for disease recurrence. Giger has proposed the formulation of predictive models, utilizing Big Data with radiomics as a lynchpin, to get the job done. This is a challenge in and of itself, as Giger told attendees at a symposium held during RSNA’s annual meeting in 2016. The reason: Predictive models incorporate a wealth of data inputs—environmental, radiological, pathological, genetic—that will create information overload if not orchestrated and harnessed.
Not that there’s any going back now. “The radiologic community needs to continue to conduct robust collection, annotation, analysis and evaluation of images of large populations,” she told attendees (reference: “Radiology’s Evolving Role in Precision Medicine,” RSNA News, Feb. 1, 2017). “We’re not going to get rid of biopsies—we want to learn from them,” Giger added, emphasizing that radiomics is, ultimately, a combination of conventional computer-aided diagnosis, deep-learning methods and human skills.
“We want to understand,” she said, “how the biopsy relates to what we’re seeing in the image.”
Those words surely constitute as precise a predictor of radiology’s unfolding role in precision medicine as is possible at this moment in the evolution of the field.