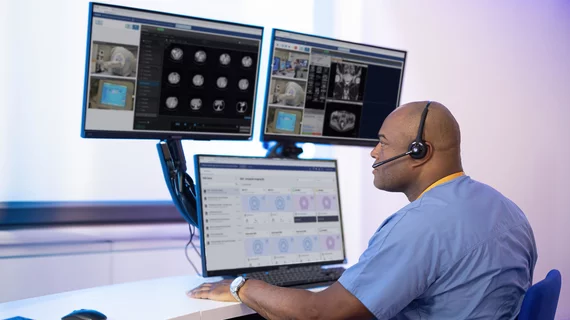
FDA clears Philips’ Radiology Operations Command Center for remote scanning
The U.S. Food and Drug Administration has cleared Philips’ Radiology Operations Command Center for use in remote scanning, the company announced recently.
The Amsterdam-based imaging giant first introduced the product in 2020 amid the pandemic, offering a vendor-neutral tool for real-time collaboration of remote care teams. Now, Philips is touting the recent FDA clearance of additional remote-scanning and protocol-adjustment features.
This will allow radiologists to assist technologists working at other sites “on the spot.” The command center also enables physicians to remotely control scans or adjust protocols to acquire higher quality images for improved diagnostic confidence.
“Healthcare providers are increasingly confronted with the challenge of not having enough skilled technologists to meet the demand for patient imaging exams, particularly for more complex exams such as cardiac MR,” Shiv Gopalkrishnan, business leader of patient care informatics at Philips, said in a Nov. 22 announcement. “With the 510(k) clearance of ROCC’s remote scanning and remote protocol management capabilities we are further empowering clinicians to deliver the timely diagnosis that patients deserve…”
Philips said the solution works with its rivals’ products and across different imaging modalities including MR or CT. A U.K. pilot study commissioned by Philips found that imaging providers achieved a 9% increase in total scanning throughput stemming from reduced exam times and other factors. ROCC also allows radiologists to edit scanner consoles in real time from any location, share expertise via voice and video, and meet HIPAA standards.
Philips’ technology upgrade comes amid growing interest in such solutions following the pandemic. The Centers for Medicare & Medicaid Services recently approved new standards related to remote scans. These are the first to require that a registered technologist always remain with the patient. Accreditors said this will ensure that qualified personnel are on-site if an emergency arises. Experts made the revisions to address “issues pertaining to staffing shortages of MRI and CT technologists,” the Intersocietal Accreditation Commission (a CMS-approved accrediting body) said on Nov. 20.
Medicare recently announced that it will allow remote monitoring of diagnostic imaging exams for at least one more year. This temporary privilege granted amid the pandemic was set to expire prior to the CMS extension. Imaging interest groups such as the Radiology Business Management Association and American College of Radiology have advocated for making the perk permanent. Some rad techs are still uncomfortable with managing imaging exams remotely, according to a recent survey from the American Society of Radiologic Technologists. More coverage is below.