Nanox shares up 63% after scoring FDA clearance for new imaging system
Nanox saw its shares rise 63% Monday morning after scoring U.S. FDA clearance for the Israel-based manufacturer’s novel imaging system.
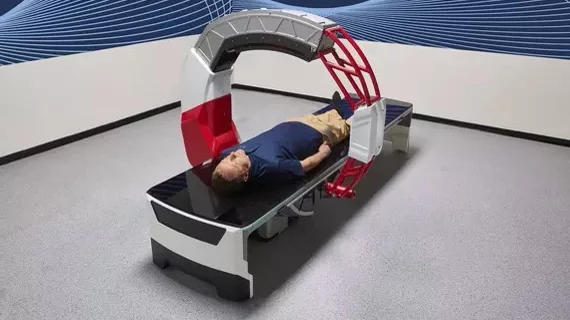
The Nanox.ARC is a stationary X-ray machine that produces images of the human musculoskeletal structure. A multi-source digital 3D tomosynthesis system, the technology utilizes novel, cold-cathode X-ray tubes and charges physicians using a “pay per scan” business model.
Nanox first applied for 510(k) clearance of its system in 2021 and during the process faced delays, investor lawsuits and an SEC investigation into its business practices.
“The FDA clearance of Nanox.ARC represents an important breakthrough and … an opportunity to increase the availability and accessibility of medical imaging in the United States and worldwide,” radiologist Geoffrey Rubin, MD, MBA, professor and chairman of the Department of Medical Imaging at the University of Arizona and a member of Nanox's Advisory Board, said in a May 1 announcement. “Medical imaging is essential for detecting, diagnosing and managing disease, guiding treatment decisions for improved health outcomes. Nanox.ARC has the potential to be a cost-effective and scalable imaging solution in healthcare settings that would otherwise be unable to deploy traditional medical imaging equipment.”
Nanox Imaging said the FDA clearance also covers marketing of the system’s accompanying cloud-based infrastructure. The administration has OK’d the Nanox.ARC for use in professional healthcare facilities or other “radiological environments.” Those include hospitals, clinics, imaging centers and medical practices, with trained radiographers, radiologists and other physicians cleared to operate the technology. In April 2021, Nanox received FDA clearance for its single-source Nanox Cart X-ray System, with the multi-source version able to reconstruct 2D projection images into a stack of slices to form a 3D visualization.
Company leaders said they’ll continue to work with the FDA to receive additional regulatory clearances, including expanding to other clinical indications. The announcement also paves the way for Nanox to seek approvals in other FDA-clearance based markets.
Nanox also previously closed the purchase of AI firm Zebra Medical Vision and teleradiology firm USARad for more than $200 million in November 2021. The company also offers a suite of artificial intelligence algorithms that augment readings of routine CT imaging.