Neuroimaging Draws a Bead on Alzheimer’s
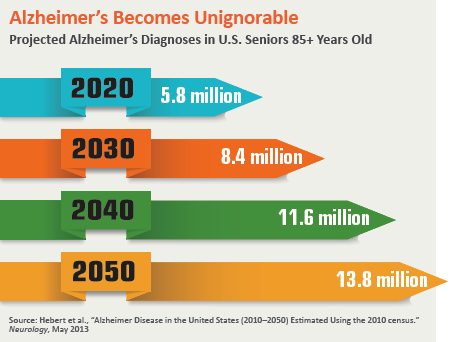
At a time when Alzheimer’s disease trials are falling like dominoes, Pfizer decided early last year to act decisively: It packed its bags and got out of town. The drug giant announced it was abandoning its extensive early-stage research to find a treatment for a disease that ravages the lives of nearly 6 million people in the U.S. alone, and would instead redirect its money to more productive investigative pursuits.
Before long, leaders of Alzheimer’s patient organizations were voicing their disappointment and alarm at the impact Pfizer’s retreat would likely have on other large pharma companies with considerable skin in the research game.
If the litany of clinical trial setbacks in recent years has cast a long shadow over chances for a breakthrough Alzheimer’s drug in the near-term, it has thrown a spotlight on the challenge and opportunity that await radiology in this high-stakes field.
The reason is clear. Because it’s so difficult now to know if someone has the biological earmarks of the disease until brain cell destruction is manifest, how do you enroll the right patients in a study designed to test a preventive drug or vaccine?
One good question deserves two more. What if a definitive way existed to identify likely Alzheimer’s candidates well in advance of symptoms? And what if this could be done while there was still an opportunity for clinicians and researchers to slow down or even arrest the debilitating disease process?
The premise that the best way to stop Alzheimer’s disease is through early diagnosis is what gives radiology such a strategically pivotal role now that now drug companies are backpedaling on their multi-billion-dollar commitment.
“I’ve been focused on imaging research for the past 20 years, and there’s no way imaging will not be the centerpiece of Alzheimer’s research in the future,” asserts Liana Apostolova, MD, professor of Alzheimer’s disease research at Indiana University School of Medicine, who was recently awarded $52 million by the NIH to study early-onset Alzheimer’s. “Blood markers are wonderful in helping us with detection, but when it comes to staging, we still need imaging.”
Imaging has in fact figured in Alzheimer’s disease detection for decades. CT and MRI scans were initially used to weed out conditions that can cause cognitive decline and memory impairment apart from dementia, which is a collection of neurodegenerative disorders of which Alzheimer’s accounts for the lion’s share.
Some 15 years ago, the University of Pittsburgh pioneered the use of PET scans to detect beta-amyloid peptide deposits, which, along with neurofibrillary tangles of tau protein, constitute the primary types of brain plaque associated with Alzheimer’s disease. The school also developed the radioactive drug Pittsburgh B to highlight those deposits in the brain.
Today neuroimaging is pushing the boundaries of Alzheimer’s research and detection further than ever before. And as it changes the contours of the disease from one defined clinically to one defined biologically, advanced imaging is considered the most hopeful platform on which any new and potentially groundbreaking therapy will take flight.
Increasingly, neuroimaging—working in tandem with biomarkers of neurodegeneration—is enabling researchers to identify the structural and functional patterns of cerebral change, as well as to visualize the molecular pathology characteristic of Alzheimer’s disease.
Just as importantly, brain imaging is helping to establish the existence of a significant preclinical and presymptomatic period during which the pathology of Alzheimer’s is detectable—and waiting for scientists to reach out and grab the brass ring of discovery.
 Predictive Power
Predictive Power
That process is well underway. In a unique but small study, researchers at the Mallinckrodt Institute of Radiology at Washington University School of Medicine in St. Louis used MRI with diffusion tensor imaging (DTI) to examine the movement of water molecules along white matter tracts of the brain. Their goal was to quantify the differences in DTI in people who decline from normal cognition to mild cognitive impairment to Alzheimer’s dementia, compared to controls who do not develop dementia.
Drawing a cohort of 61 patients from the Alzheimer’s Disease Neuroimaging Initiative, the team found that patients who developed Alzheimer’s had lower measures of fractional anisotropy (FA), a gauge of water-molecule movement, suggesting damage to white matter tracts.
In fact, the study was able to predict with 89 percent accuracy which patients would progress to Alzheimer’s using FA values and other associated global metrics of white matter integrity.
By comparison, common predictive models like standardized questionnaires used to measure cognition and genetic testing for APOE-e4, the strongest Alzheimer’s risk gene, have accuracy rates of around 70 percent.
Of paramount importance, the MRI scans with DTI were able to predict dementia an average of 2.6 years before memory loss became clinically detectable.
“This makes it possible to develop MRI protocols to screen individuals who might be at risk for cognitive decline in the future even if they don’t have any memory loss symptoms,” says study co-author Cyrus Raji, MD, PhD, a Mallinckrodt Institute neuroradiologist who’s a pioneering radiology researcher with p articular i nterest in Alzheimer’s. “That’s important because drug companies would then have better populations to test promising treatments on.”
Raji foresees a future where an individual with a family history of dementia or Alzheimer’s or someone whose genetic test results from 23andMe, say, showed a predisposition to the disease to get scanned and baselined to determine if they have any subtle or quantitative signs of future pathology.
 AI Enters the Alzheimer’s Arena
AI Enters the Alzheimer’s Arena
Artificial intelligence (AI) and deep learning (DL) also hold extraordinary potential to turn imaging into a more powerful prognostic tool for Alzheimer’s disease. And the recent development of a DL algorithm that achieved 100 percent sensitivity on a small cohort of patients at detecting the disease an average of more than six years prior to final diagnosis provides a revealing and highly encouraging look into the future.
Researchers in the Radiology and Biomedical Imaging Department of UC-San Francisco (UCSF) trained the algorithm using an 18-F-fluorodeoxyglucose PET (FDG-PET) scan, which shows changes in brain metabolism as highlighted by glucose uptake in certain regions of the brain. They describe their work in “A Deep Learning Model to Predict a Diagnosis of Alzheimer Disease by Using 18F-FDG PET of the Brain,” published online last November in Radiology.
But as corresponding author Jae Ho Sohn, MD, explains to RBJ, detecting subtle and diffuse differences in patterns of glucose uptake in the brain is not a task humans are particularly adept at. The process becomes even more challenging, he adds, in a disease like Alzheimer’s with a wide and ever-changing clinical spectrum from normal cognition to mild cognitive impairment to Alzheimer’s, in which only a fraction of patients with memory issues advance to Alzheimer’s.
Approached by faculty advisor and senior study author Benjamin Franc, MD, a nuclear medicine specialist at UCSF, to find a solution, Sohn and a team of engineers he assembled from UC-Berkeley used their AI expertise to address the sheer volume and complexity of the FDG PET data. More specifically, they trained their DL algorithm on a dataset of more than 2,100 FDG PET brain images collected from the Alzheimer’s Disease Neuroimaging Initiative. After testing the algorithm on an independent set of 40 imaging exams from patients they had never before studied, the researchers were elated over its performance.
While cautioning that the algorithm needs further validation from a large prospective study, Sohn maintains “it can become a useful detection tool in the hands of radiologists, especially in conjunction with other biochemical and imaging tests,” including MRI and PET scans with specific radiotracers.
It also could prove cost-effective in ways that could enfranchise vastly greater numbers of patients. Indeed, among the biomarkers science has produced in recent years for early diagnosis of Alzheimer’s disease are beta-amyloid in the cerebral spinal fluid and PET imaging radiolabeled beta-amyloid ligands such as F-florbetapir, flutemetamol and florbetaben.
However, as Sohn points out in his research, these innovations are associated with high costs that may not be reimbursable by patients’ health insurance or, for that matter, may not be universally available in healthcare settings. Injecting a deep learning algorithm into the mix, however, could offer the opportunity to “provide clinically relevant molecular imaging data across a multitude of populations and settings worldwide,” says Sohn.
More Machine Learning, Please
In the view of neuroradiologist Mykol Larvie, MD, of the Cleveland Clinic, there is an “inevitability” to the deployment of machine learning and other advanced computational methods within radiology, as he outlined in an opinion piece accompanying the aforementioned study by Sohn and colleagues in Radiology.
“Many proof-of-principle studies have demonstrated that machine learning algorithms outperform human experts under highly selective and constrained conditions, notably with certain radiology tasks,” he wrote in the commentary.
As for its applicability to Alzheimer’s disease, a machine learning model “would have the advantage of simultaneously analyzing, say, an MRI scan and a PET scan and clinical data,” Larvie tells RBJ, “and then relating them to a potentially very large database of normative data so that the changes of normal aging could be compared to the changes of neurodegeneration.”
Insights from machine learning could be particularly instructive, says Larvie, when applied to the FDG PET scan, a technology he regards as “tremendously useful and very revealing, especially in neurodegenerative disease.”
And by the way, he adds, “it’s cheap,” at about $100 a dose compared to about $3,500 for a beta-amyloid radiotracer.
Despite these advantages, Larvie notes, FDG PET suffers from a fairly high degree of variability in interpretation of scans from one radiology practice to the next, depending on the reader’s skills and training. Improvement is sorely needed, he says.
Machine learning, for its part, could help fill that interpretive void, given its computational strengths and a methodology that’s well-suited to the classification of PET data.
“If a machine can get you that far,” Larvie says, “then a physician looking at the patient’s other medical records, like structured cognitive testing data, could come up with a more reliable diagnosis than anyone looking at just a single data set.”
To Nudge the Druggist
But how meaningful are diagnoses, including those that are both early and highly accurate, given the continued absence of an effective Alzheimer’s treatment?
Researchers in the field admit they are frustrated and often disheartened by the vacuum, but view their ongoing work as critical to turning the tide and keeping the pharmaceutical industry engaged.
“The research side of me is becoming more and more committed to finding something by virtue of the patients who continue to come to our clinics asking about the new drug on the horizon, and I continue to tell them there’s nothing yet,” says Apostolova, who is currently enrolling as part of her large NIH study 500 individuals who have shown through amyloid PET scans evidence of early onset Alzheimer’s disease.
By drawing on people in their 30s, 40s and 50s who have already seen their family lives disrupted and careers jeopardized by a disease that begs for a therapeutic way to control or cure it, Apostolova believes her study will have a powerful impact on and help to improve the mindset of drug companies that may otherwise be getting cold feet.
“We’ve already seen interest from several pharmaceutical companies that are willing to work with us,” she says. “We hope in a couple of years to be deciding which [of their] agents to use.”
Neuroradiologists like Sohn and Raji are not only eager to proceed with their novel research but also confident that, if and when a therapy emerges to reverse or tame Alzheimer’s, they and the rest of their field will be prepared to guide and nurture it.
“Because imaging has been so far ahead of treatment development, when something is available that’s innovative and actually works, we’ll be ready to track individuals who are at risk and ready to study patients who should benefit from the treatment and evaluate their response,” says Raji. “We are very well positioned for this important role.”
