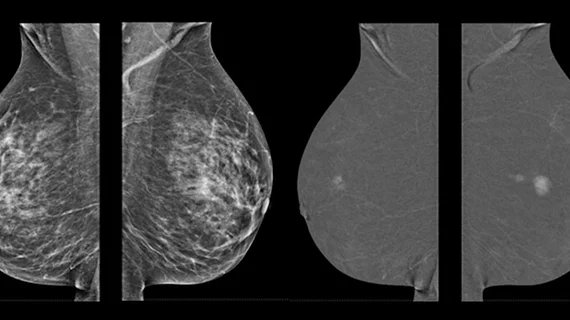
3D mammography approaching 50% of breast imaging systems in the U.S.
The latest U.S. Food and Drug Administration data on mammography systems installed nationwide shows digital breast tomosynthesis (DBT) systems are rapidly replacing traditional 2D full field digital mammography (FFDM) units.
The FDA collects data on breast imaging systems from the Mammography Quality Standards Act program, responsible for quality assurance inspections to certify breast imaging systems.
The latest FDA statistics from March 1 show 10,744 DBT systems now in service, which is about 45% of U.S. mammography systems. Of the total of 23,941 mammography systems in service, 55% (13,192) are still FFDM systems. However, the number of DBT systems has been steadily rising over the past few years.
Most breast imaging centers now have one or more 3D mammography systems to image women with dense breasts or for more detailed images on callback exams. Of the 8,753 facilities performing breast imaging, 7,158 have at least one DBT system.
The advantage of DBT systems is that they create a dataset of images that can be scrolled through slice-by-slice similar to computed tomography. This allows radiologists to determine if areas that appear like a cancer lesion are the real thing or just areas of dense breast tissue stacked up. Advocates for 3D mammography systems say the technology can help reduce the number of biopsies and imaging recall exams compared to 2D mammography. However, the systems are more expensive, require much more data storage for the larger image files and take longer for radiologists to read.
How many U.S. mammograms are performed each year?
FDA data reports there have been more than 39 million mammography exams performed over the past year, between February 2021 and last month.
MQSA inspection statistics show majority of mammography centers are in compliance with FDA standards
Fiscal year 2022 inspection statistics as of March 1 also reported 3,237 breast imaging facilities were inspected, including 8,806 individual imaging systems.
The FDA reports 86.8% of breast imaging centers had no violations in Mammography Quality Standards Act reporting. For the facilities that did have a noncompliance issue, 0.8% had a Level 1 violation, and 12.3% had a Level 2 violation
MQSA re-accreditation of breast imaging facilities takes place once every three years.
Learn more about the FDA breast imaging quality inspections.
Related Breast Imaging Content:
VIDEO: 3D mammography is becoming the standard-of-care in breast imaging — Interview with John Lewin, MD
Q&A: Should COVID vaccinated patients delay getting breast imaging — new study says no
FDA issues new guidance on appealing decisions that adversely impact mammography accreditation
Breast cancer is over diagnosed in 15% of screenings
FDA issues new guidance on appealing decisions that adversely impact mammography accreditation
Breast MRI screening cuts cancer mortality rates in half for women with lesser-known gene mutations
Combining neural network with breast density measurements boosts interval cancer detection rates
Mammograms should not be delayed after COVID vaccine, research shows
DBT plus synthesized mammography drops patients’ dosage without losing imaging quality